| [1] |
ZHOU MG, WANG HD, ZENG XY, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394( 10204): 1145- 1158. DOI: 10.1016/S0140-6736(19)30427-1. |
| [2] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. |
| [3] |
General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. |
| [4] |
ZHU HD, LI HL, HUANG MS, et al. Transarterial chemoembolization with PD-(L) 1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma(CHANCE001)[J]. Signal Transduct Target Ther, 2023, 8( 1): 58. DOI: 10.1038/s41392-022-01235-0. |
| [5] |
SANGRO B, KUDO M, QIN S, et al. A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1[J]. Ann Oncol, 2020, 31: S202- S203. DOI: 10.1016/j.annonc.2020.04.429. |
| [6] |
XIN YJ, ZHANG XY, LIU N, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma[J]. Hepatol Int, 2023, 17( 3): 753- 764. DOI: 10.1007/s12072-023-10502-3. |
| [7] |
Liver Oncology Branch, China Association for the Promotion of International Exchange in Healthcare; Branch Immunology, China Association for the Promotion of International Exchange in Healthcare; Cooperative Group for Chinese Expert Consensus on Targeted Immunotherapy Combined with Local Therapy for Advanced Hepatocellular Cancer. Chinese expert consensus on targeted immunotherapy combined with local therapy for advanced hepatocellular cancer[J]. J Clin Hepatol, 2023, 39( 12): 2782- 2792. DOI: 10.3969/j.issn.1001-5256.2023.12.006. |
| [8] |
HIRAOKA A, KUMADA T, KUDO M, et al. Albumin-bilirubin(ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan society of hepatology: A comparison with the liver damage and Child-pugh classifications[J]. Liver Cancer, 2017, 6( 3): 204- 215. DOI: 10.1159/000452846. |
| [9] |
JOHNSON PJ, BERHANE S, KAGEBAYASHI C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade[J]. J Clin Oncol, 2015, 33( 6): 550- 558. DOI: 10.1200/JCO.2014.57.9151. |
| [10] |
WANG YY, ZHONG JH, SU ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma[J]. Br J Surg, 2016, 103( 6): 725- 734. DOI: 10.1002/bjs.10095. |
| [11] |
WANG ZX, WANG EX, XIA DD, et al. Value of Child-Pugh score versus albumin-bilirubin grade in predicting the prognosis of unresectable hepatocellular carcinoma treated by transarterial chemoembolization[J]. J Clin Hepatol, 2020, 36( 1): 113- 117. DOI: 10.3969/j.issn.1001-5256.2020.01.025. |
| [12] |
KUMADA T, TOYODA H, TADA T, et al. Changes in background liver function in patients with hepatocellular carcinoma over 30 years: Comparison of child-pugh classification and albumin bilirubin grade[J]. Liver Cancer, 2020, 9( 5): 518- 528. DOI: 10.1159/000507933. |
| [13] |
SUN Y, ZHANG HH, SHENG SP, et al. Prognostic significance of albumin-bilirubin grading in patients with early hepatocellular carcinoma after radiofrequency ablation treatment[J]. J Interv Radiol, 2021, 30( 5): 502- 507. DOI: 10.3969/j.issn.1008-794X.2021.05.018. |
| [14] |
LEE IC, HUNG YW, LIU CA, et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma[J]. Liver Int, 2019, 39( 9): 1704- 1712. DOI: 10.1111/liv.14194. |
| [15] |
SAITO N, NISHIOFUKU H, SATO T, et al. Predictive factors of complete response to transarterial chemoembolization in intermediate stage hepatocellular carcinoma beyond up-to-7 criteria[J]. Cancers, 2023, 15( 9): 2609. DOI: 10.3390/cancers15092609. |
| [16] |
HICKEY R, MOULI S, KULIK L, et al. Independent analysis of albumin-bilirubin grade in a 765-patient cohort treated with transarterial locoregional therapy for hepatocellular carcinoma[J]. J Vasc Interv Radiol, 2016, 27( 6): 795- 802. DOI: 10.1016/j.jvir.2016.03.005. |
| [17] |
YU WM, HONG WQ, SUN BL, et al. Value of albumin-bilirubin grade in predicting liver function changes and prognosis of hepatocellular carcinoma patients undergoing transarterial chemoembolization: A Meta-analysis[J]. J Clin Hepatol, 2021, 37( 11): 2575- 2583. DOI: 10.3969/j.issn.1001-5256.2021.11.018. |
| [18] |
HIRAOKA A, KUMADA T, TSUJI K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis[J]. Liver Cancer, 2019, 8( 2): 121- 129. DOI: 10.1159/000488778. |
| [19] |
PINATO DJ, KANEKO T, SAEED A, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: Adjunctive role of the ALBI grade[J]. Cancers, 2020, 12( 7): 1862. DOI: 10.3390/cancers12071862. |
| [20] |
KUDO M, FINN RS, CHENG AL, et al. Albumin-bilirubin grade analyses of atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma: A post hoc analysis of the Phase III IMbrave150 study[J]. Liver Cancer, 2023, 12( 5): 479- 493. DOI: 10.1159/000529996. |
| [21] |
TANAKA K, TSUJI K, HIRAOKA A, et al. Usefulness of tumor marker score for predicting the prognosis of hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: A multicenter retrospective study[J]. Cancers, 2023, 15( 17): 4348. DOI: 10.3390/cancers15174348. |
| [22] |
KELLEY RK, RIMASSA L, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma(COSMIC-312): A multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23( 8): 995- 1008. DOI: 10.1016/S1470-2045(22)00326-6. |
| [23] |
KUDO M, GALLE PR, BRANDI G, et al. Effect of ramucirumab on ALBI grade in patients with advanced HCC: Results from REACH and REACH-2[J]. JHEP Rep, 2021, 3( 2): 100215. DOI: 10.1016/j.jhepr.2020.100215. |
| [24] |
LEE PC, CHEN YT, CHAO YE, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma[J]. Liver Int, 2018, 38( 2): 321- 330. DOI: 10.1111/liv.13527. |
| [25] |
KUDO M. Newly developed modified ALBI grade shows better prognostic and predictive value for hepatocellular carcinoma[J]. Liver Cancer, 2022, 11( 1): 1- 8. DOI: 10.1159/000521374. |
| [26] |
Clinical Guidelines Committee of Chinese College of Interventionalists. Expert consensus on the TACE failure/refractoriness and its subsequent therapies in treating patients with hepatocellular carcinoma[J]. J Interv Radiol, 2022, 31( 11): 1039- 1044. DOI: 10.3969/j.issn.1008-794X.2022.11.001. |
| [27] |
LENCIONI R, LLOVET JM. Modified RECIST(mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30( 1): 52- 60. DOI: 10.1055/s-0030-1247132. |
| [28] |
PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. |
| [29] |
HSU WF, HSU SC, CHEN TH, et al. Modified albumin-bilirubin model for stratifying survival in patients with hepatocellular carcinoma receiving anticancer therapy[J]. Cancers, 2022, 14( 20): 5083. DOI: 10.3390/cancers14205083. |
| [30] |
IIJIMA H, KUDO M, KUBO S, et al. Report of the 23 rd nationwide follow-up survey of primary liver cancer in Japan(2014-2015)[J]. Hepatol Res, 2023, 53( 10): 895- 959. DOI: 10.1111/hepr.13953. |
| [31] |
AMIOKA K, KAWAOKA T, KINAMI T, et al. Analysis of lenvatinib’s efficacy against intermediate-stage unresectable hepatocellular carcinoma[J]. Cancers, 2022, 14( 20): 5066. DOI: 10.3390/cancers14205066. |
| [32] |
REDDY KR, MCLERRAN D, MARSH T, et al. Incidence and risk factors for hepatocellular carcinoma in cirrhosis: The multicenter hepatocellular carcinoma early detection strategy(HEDS) study[J]. Gastroenterology, 2023, 165( 4): 1053- 1063. DOI: 10.1053/j.gastro.2023.06.027. |
| [33] |
REIG M, FORNER A, RIMOLA J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update[J]. J Hepatol, 2022, 76( 3): 681- 693. DOI: 10.1016/j.jhep.2021.11.018. |



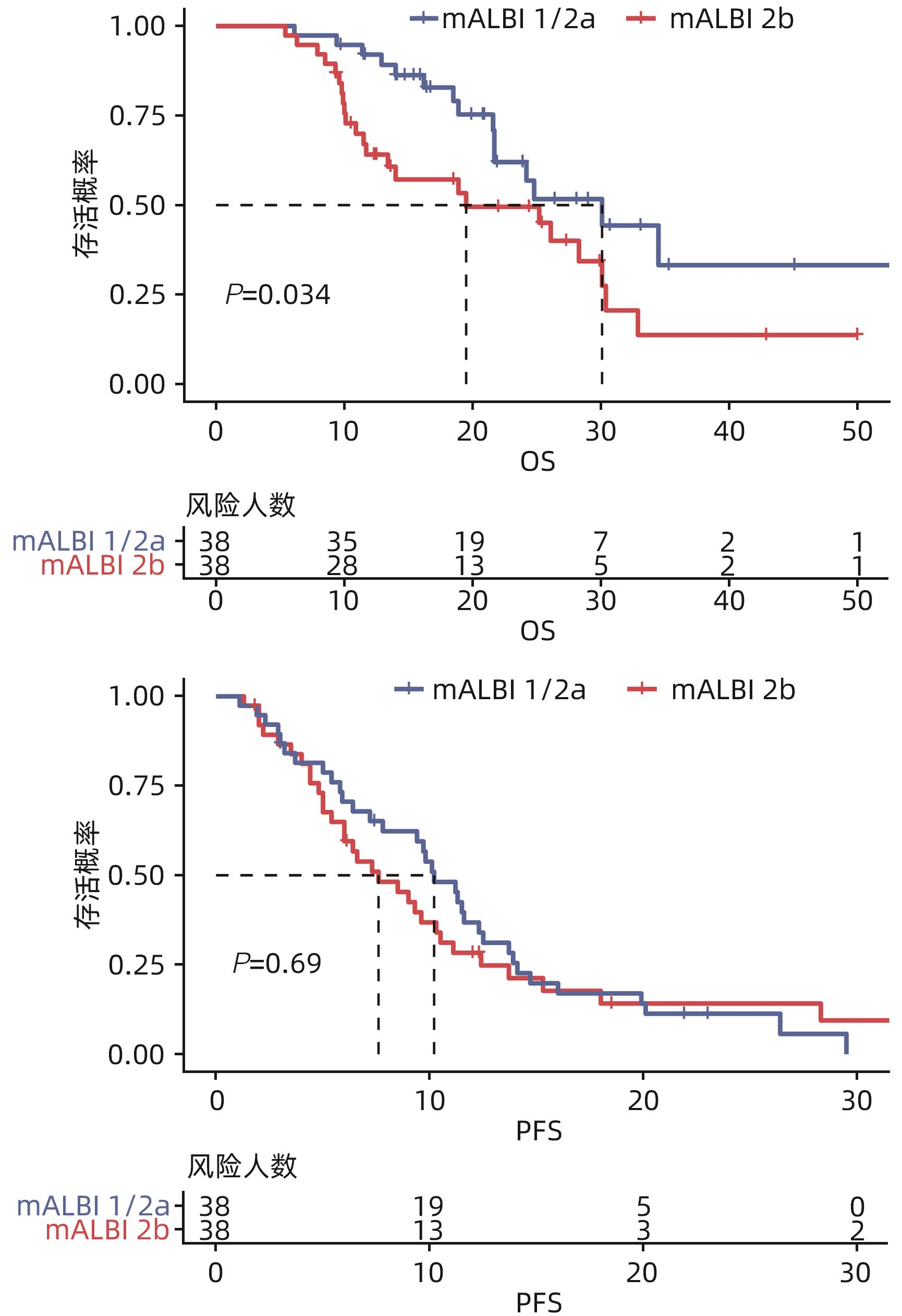




 DownLoad:
DownLoad: