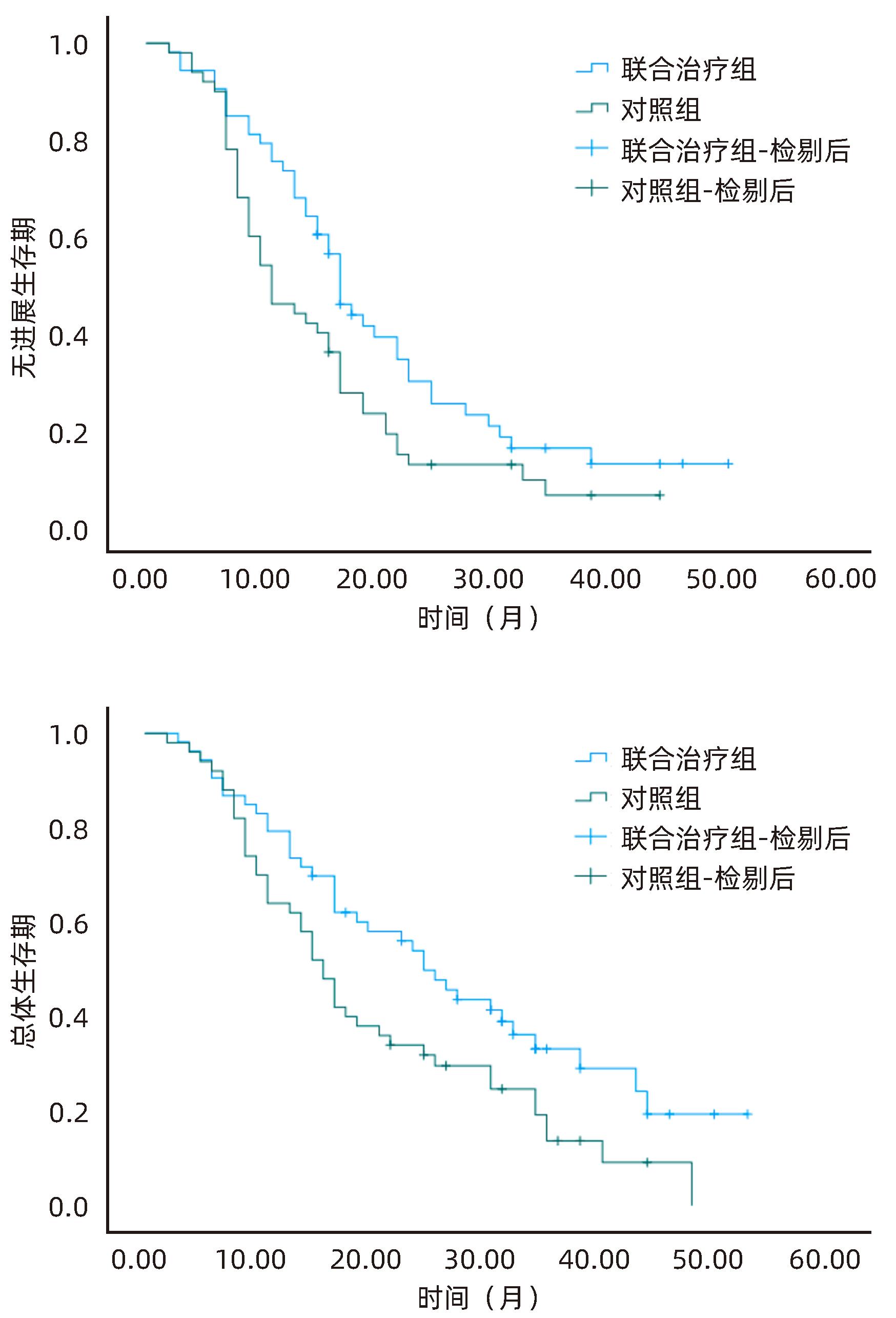
| [1] |
RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. |
| [2] |
WANG T, WANG CY, LIU JY, et al. Effectiveness and safety of ultrasound-guided ablation in treatment of primary liver cancer in dangerous areas[J]. J Clin Hepatol, 2021, 37( 7): 1594- 1598. DOI: 10.3969/j.issn.1001-5256.2021.07.023. |
| [3] |
MAO B, MA JD, DUAN SB, et al. Preoperative classification of primary and metastatic liver cancer via machine learning-based ultrasound radiomics[J]. Eur Radiol, 2021, 31( 7): 4576- 4586. DOI: 10.1007/s00330-020-07562-6. |
| [4] |
ZHAI HG, XIAO ZH, YANG SL. Effect of CT-guided cryoablation with Argon-helium cryoablation on hepatic blood flow changes in primary liver cancer[J]. Chin J CT MRI, 2021, 19( 9): 96- 99, 110. DOI: 10.3969/j.issn.1672-5131.2021.09.030. |
| [5] |
LI X, MA LN, CHENG XZ, et al. Effect and safety of chemotherapy-based PD-1 inhibitors in the treatment of advanced lung adenocarcinoma[J]. J Pract Med, 2021, 37( 3): 365- 368. DOI: 10.3969/j.issn.1006-5725.2021.03.018. |
| [6] |
WANG Y, LUO D, LEI M, et al. Effect of silencing PD-1 gene on AAV8-mediated T cell immune response in HBV-infected rats[J]. Chin J Nosocomiology, 2021, 31( 17): 2584- 2588.
王燕, 罗丹, 雷敏, 等. 沉默PD-1基因对AAV8介导的HBV感染大鼠T细胞免疫应答的影响[J]. 中华医院感染学杂志, 2021, 31( 17): 2584- 2588.
|
| [7] |
WEYKAMP F, HOEGEN P, REGNERY S, et al. Long-term clinical results of MR-guided stereotactic body radiotherapy of liver metastases[J]. Cancers(Basel), 2023, 15( 10): 2786. DOI: 10.3390/cancers15102786. |
| [8] |
XU HC, WANG FL, XIE LH. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024. |
| [9] |
LIU Y, ZHENG JX, HAO JL, et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019[J]. Cancer Med, 2022, 11( 5): 1310- 1323. DOI: 10.1002/cam4.4551. |
| [10] |
LUO J, LYU CH, YANG YP. Clinical efficacy and safety of percutaneous cryoablation combined with percutaneous ethanol injection in elderly patients with hepatocellular carcinoma aged 70 years or older[J]. J Clin Hepatol, 2022, 38( 2): 365- 371. DOI: 10.3969/j.issn.1001-5256.2022.02.021. |
| [11] |
JIN ZQ, LIU YX, LIANG M, et al. Comparison of efficacy and safety between cryoballoon ablation and radiofrequency catheter ablation in the treatment of paroxysmal atrial fibrillation[J]. Clin J Med Offic, 2021, 49( 10): 1079- 1082. DOI: 10.16680/j.1671-3826.2021.10.05. |
| [12] |
MA JB, WANG FM, ZHANG WQ, et al. Percutaneous cryoablation for the treatment of liver cancer at special sites: An assessment of efficacy and safety[J]. Quant Imaging Med Surg, 2019, 9( 12): 1948- 1957. DOI: 10.21037/qims.2019.11.12. |
| [13] |
DONNE R, LUJAMBIO A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma[J]. Hepatology, 2023, 77( 5): 1773- 1796. DOI: 10.1002/hep.32740. |
| [14] |
MERELLI B, MASSI D, CATTANEO L, et al. Targeting the PD1/PD-L1 axis in melanoma: Biological rationale, clinical challenges and opportunities[J]. Crit Rev Oncol Hematol, 2014, 89( 1): 140- 165. DOI: 10.1016/j.critrevonc.2013.08.002. |
| [15] |
HEINRICH S, CRAIG AJ, MA LC, et al. Understanding tumour cell heterogeneity and its implication for immunotherapy in liver cancer using single-cell analysis[J]. J Hepatol, 2021, 74( 3): 700- 715. DOI: 10.1016/j.jhep.2020.11.036. |
| [16] |
FREEMAN GJ, LONG AJ, IWAI Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation[J]. J Exp Med, 2000, 192( 7): 1027- 1034. DOI: 10.1084/jem.192.7.1027. |
| [17] |
TOPALIAN SL, HODI FS, BRAHMER JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. N Engl J Med, 2012, 366( 26): 2443- 2454. DOI: 10.1056/nejmoa1200690. |
| [18] |
FINN RS, ZHU AX. Evolution of systemic therapy for hepatocellular carcinoma[J]. Hepatology, 2021, 73( Suppl 1): 150- 157. DOI: 10.1002/hep.31306. |
| [19] |
CHEN EB, YI J, JIANG J, et al. Identification and validation of a fatty acid metabolism-related lncRNA signature as a predictor for prognosis and immunotherapy in patients with liver cancer[J]. BMC Cancer, 2022, 22( 1): 1037. DOI: 10.1186/s12885-022-10122-4. |
| [20] |
LAI FT. Clinical efficacy and safety of carrizumab in the treatment of advanced primary liver cancer[J]. Shanxi Med J, 2021, 50( 15): 2269- 2272.
赖奉庭. 卡瑞利珠单抗治疗晚期原发性肝癌临床效果及安全性研究[J]. 山西医药杂志, 2021, 50( 15): 2269- 2272.
|
| [21] |
LEE YY, LUO SC, LEE CH, et al. Optimizing tumor-associated antigen-stimulated autologous dendritic cell and cytokine-induced killer cell coculture to enhance cytotoxicity for cancer immunotherapy in manufacturing[J]. BMC Immunol, 2023, 24( 1): 14. DOI: 10.1186/s12865-023-00552-5. |



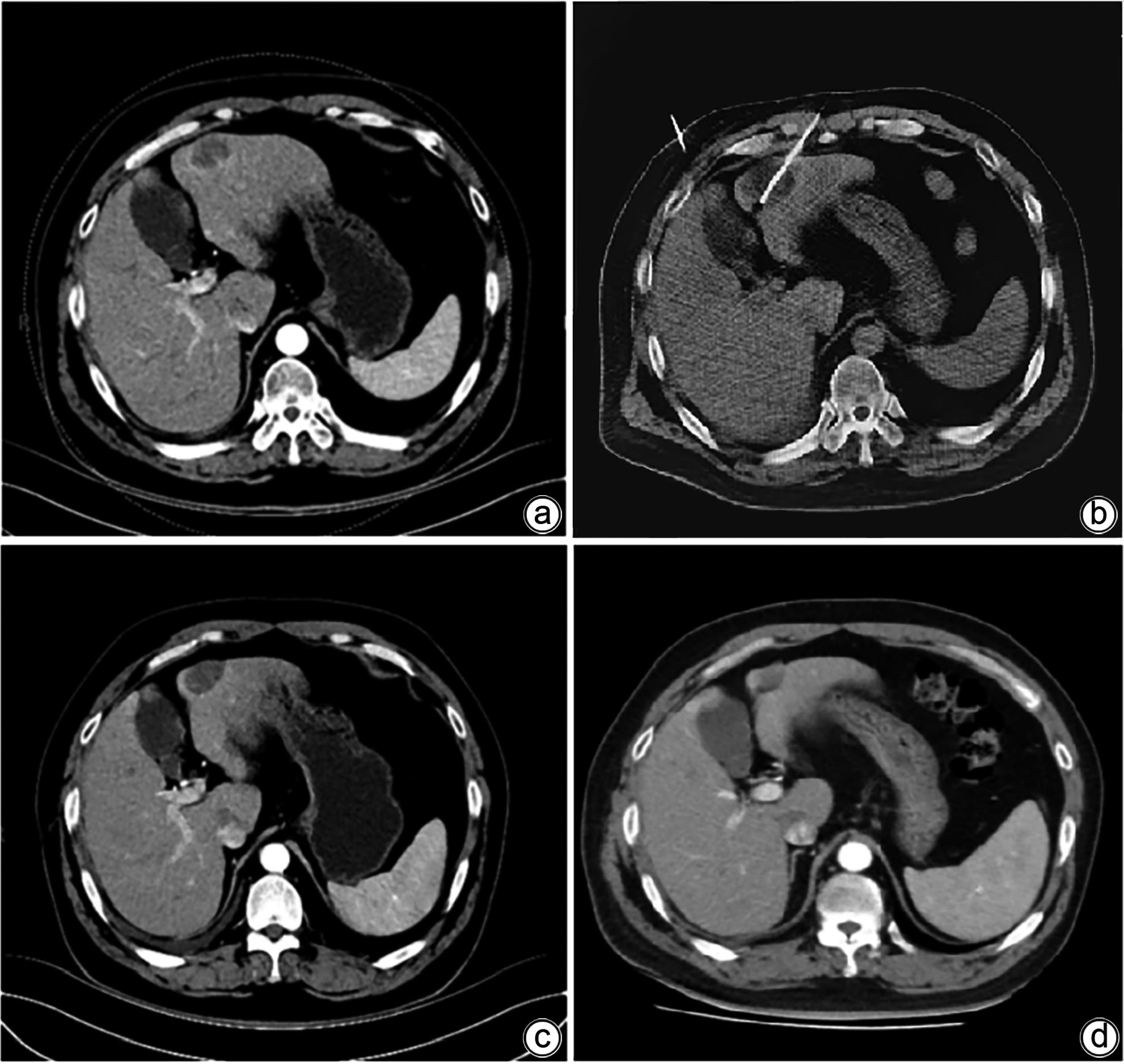




 DownLoad:
DownLoad: