| [1] |
LOOMIS D, GROSSE Y, LAUBY-SECRETAN B, et al. The carcinogenicity of outdoor air pollution[J]. Lancet Oncol, 2013, 14(13): 1262-1263. DOI: 10.1016/s1470-2045(13)70487-x. |
| [2] |
VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. |
| [3] |
|
| [4] |
YAN YC, WEN K, MAO K, et al. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36(10): 2167-2172. DOI: 10.3969/j.issn.1001-5256.2020.10.002. |
| [5] |
YUE H, YUN Y, GAO R, et al. Winter polycyclic aromatic hydrocarbon-bound particulate matter from peri-urban north China promotes lung cancer cell metastasis[J]. Environ Sci Technol, 2015, 49(24): 14484-14493. DOI: 10.1021/es506280c. |
| [6] |
KREYLING WG, HIRN S, MÖLLER W, et al. Air-blood barrier translocation of tracheally instilled gold nanoparticles inversely depends on particle size[J]. ACS Nano, 2014, 8(1): 222-233. DOI: 10.1021/nn403256v. |
| [7] |
GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2018, 392(10159): 1923-1994. DOI: 10.1016/S0140-6736(18)32225-6. |
| [8] |
HONG Z, GUO Z, ZHANG R, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells[J]. Tohoku J Exp Med, 2016, 239(2): 117-125. DOI: 10.1620/tjem.239.117. |
| [9] |
DENG X, RUI W, ZHANG F, et al. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells[J]. Cell Biol Toxicol, 2013, 29(3): 143-157. DOI: 10.1007/s10565-013-9242-5. |
| [10] |
VAN EEDEN SF, TAN WC, SUWA T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10))[J]. Am J Respir Crit Care Med, 2001, 164(5): 826-830. DOI: 10.1164/ajrccm.164.5.2010160. |
| [11] |
ZHANG Y, WANG S, ZHU J, et al. Effect of atmospheric PM2.5 on expression levels of NF-κB genes and inflammatory cytokines regulated by NF-κB in human macrophage[J]. Inflammation, 2018, 41(3): 784-794. DOI: 10.1007/s10753-018-0732-8. |
| [12] |
KAWANISHI M, FUJIKAWA Y, ISHII H, et al. Adduct formation and repair, and translesion DNA synthesis across the adducts in human cells exposed to 3-nitrobenzanthrone[J]. Mutat Res, 2013, 753(2): 93-100. DOI: 10.1016/j.mrgentox.2013.03.005. |
| [13] |
MEHTA M, CHEN LC, GORDON T, et al. Particulate matter inhibits DNA repair and enhances mutagenesis[J]. Mutat Res, 2008, 657(2): 116-121. DOI: 10.1016/j.mrgentox.2008.08.015. |
| [14] |
ZHOU Q, WANG L, CAO Z, et al. Dispersion of atmospheric fine particulate matters in simulated lung fluid and their effects on model cell membranes[J]. Sci Total Environ, 2016, 542(Pt A): 36-43. DOI: 10.1016/j.scitotenv.2015.10.083. |
| [15] |
CONKLIN DJ. From lung to liver: How does airborne particulate matter trigger NASH and systemic insulin resistance?[J]. J Hepatol, 2013, 58(1): 8-10. DOI: 10.1016/j.jhep.2012.10.008. |
| [16] |
PAN WC, WU CD, CHEN MJ, et al. Fine particle pollution, alanine transaminase, and liver cancer: A Taiwanese Prospective Cohort Study (REVEAL-HBV)[J]. J Natl Cancer Inst, 2016, 108(3): djv341. DOI: 10.1093/jnci/djv341. |
| [17] |
PEDERSEN M, ANDERSEN ZJ, STAFOGGIA M, et al. Ambient air pollution and primary liver cancer incidence in four European cohorts within the ESCAPE project[J]. Environ Res, 2017, 154: 226-233. DOI: 10.1016/j.envres.2017.01.006. |
| [18] |
DENG H, ECKEL SP, LIU L, et al. Particulate matter air pollution and liver cancer survival[J]. Int J Cancer, 2017, 141(4): 744-749. DOI: 10.1002/ijc.30779. |
| [19] |
LEE CH, HSIEH SY, HUANG WH, et al. Association between ambient particulate matter 2.5 exposure and mortality in patients with hepatocellular carcinoma[J]. Int J Environ Res Public Health, 2019, 16(14): 2490. DOI: 10.3390/ijerph16142490. |
| [20] |
ZHANG Q, LUO Q, YUAN X, et al. Atmospheric particulate matter2.5 promotes the migration and invasion of hepatocellular carcinoma cells[J]. Oncol Lett, 2017, 13(5): 3445-3450. DOI: 10.3892/ol.2017.5947. |
| [21] |
CAVE M, APPANA S, PATEL M, et al. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004[J]. Environ Health Perspect, 2010, 118(12): 1735-1742. DOI: 10.1289/ehp.1002720. |
| [22] |
KIM KN, LEE H, KIM JH, et al. Physical activity- and alcohol-dependent association between air pollution exposure and elevated liver enzyme levels: An elderly panel study[J]. J Prev Med Public Health, 2015, 48(3): 151-169. DOI: 10.3961/jpmph.15.014. |
| [23] |
LI R, ZHANG M, WANG Y, et al. Effects of sub-chronic exposure to atmospheric PM2.5 on fibrosis, inflammation, endoplasmic reticulum stress and apoptosis in the livers of rats[J]. Toxicol Res (Camb), 2018, 7(2): 271-282. DOI: 10.1039/c7tx00262a. |
| [24] |
|
| [25] |
ZHOU W, TIAN D, HE J, et al. Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation[J]. Oncotarget, 2016, 7(15): 20691-20703. DOI: 10.18632/oncotarget.7842. |
| [26] |
VELASCO G. Endoplasmic reticulum stressed by pollution. Focus on "Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues"[J]. Am J Physiol Cell Physiol, 2010, 299(4): C727-C728. DOI: 10.1152/ajpcell.00271.2010. |
| [27] |
HABERZETTL P, O'TOOLE TE, BHATNAGAR A, et al. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress[J]. Environ Health Perspect, 2016, 124(12): 1830-1839. DOI: 10.1289/EHP212. |
| [28] |
XU J, ZHANG W, LU Z, et al. Airborne PM2.5-induced hepatic insulin resistance by Nrf2/JNK-mediated signaling pathway[J]. Int J Environ Res Public Health, 2017, 14(7): 787. DOI: 10.3390/ijerph14070787. |
| [29] |
TAN HH, FIEL MI, SUN Q, et al. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease[J]. J Immunotoxicol, 2009, 6(4): 266-275. DOI: 10.1080/15476910903241704. |
| [30] |
|
| [31] |
AFFO S, YU LX, SCHWABE RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer[J]. Annu Rev Pathol, 2017, 12: 153-186. DOI: 10.1146/annurev-pathol-052016-100322. |
| [32] |
ZHENG Z, ZHANG X, WANG J, et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models[J]. J Hepatol, 2015, 63(6): 1397-1404. DOI: 10.1016/j.jhep.2015.07.020. |
| [33] |
QIU YN, WANG GH, ZHOU F, et al. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy[J]. Ecotoxicol Environ Saf, 2019, 167: 178-187. DOI: 10.1016/j.ecoenv.2018.08.050. |
| [34] |
JIN D, TAO J, LI D, et al. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion[J]. Oncotarget, 2015, 6(32): 33523-33533. DOI: 10.18632/oncotarget.5590. |
| [35] |
ZHANG H, LI Z. microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma[J]. Open Med (Wars), 2019, 14: 673-682. DOI: 10.1515/med-2019-0078. |
| [36] |
YANG ZE, ZHAO JK, YU HY. Current research on the biological function of mesenchymal stem cell-derived exosome and its regulatory effect on tumor[J]. Clin J Med Offic, 2020, 48(11): 1386-1388. DOI: 10.16680/j.1671-3826.2020.11.47. |
| [37] |
LI YG, DENG TX, ZHAO KF, et al. Effects of stable overexpression of TFF1 on proliferation, invasion, migration and EMT of MHCC97H hepatic cancer cell[J]. Chin J Immunol, 2021, 37(14): 1733-1737. DOI: 10.3969/j.issn.1000-484X.2021.14.013. |
| [38] |
WU WL, WANG WY, YAO WQ, et al. Suppressive effects of microRNA-16 on the proliferation, invasion and metastasis of hepatocellular carcinoma cells[J]. Int J Mol Med, 2015, 36(6): 1713-1719. DOI: 10.3892/ijmm.2015.2379. |
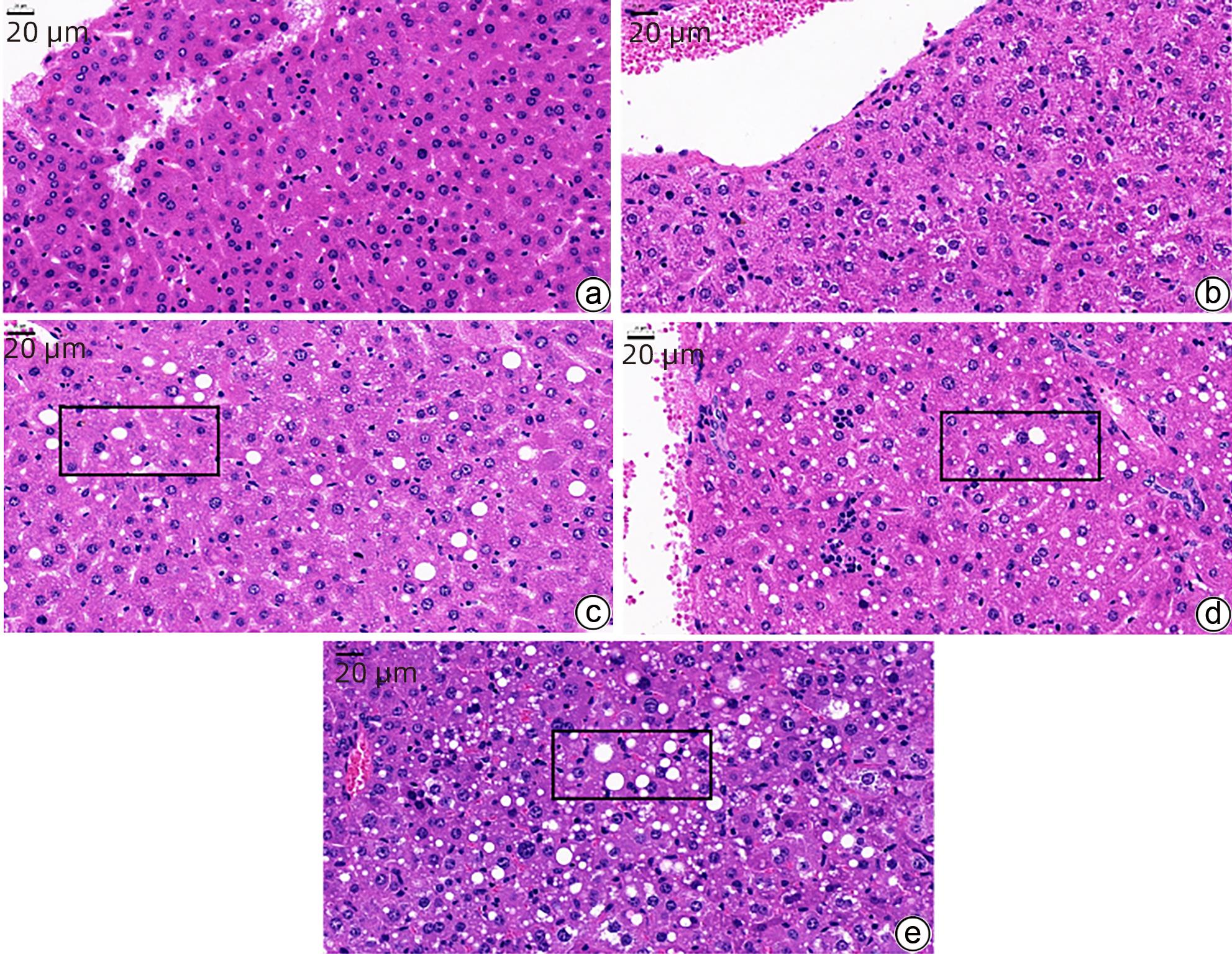








 下载:
下载:
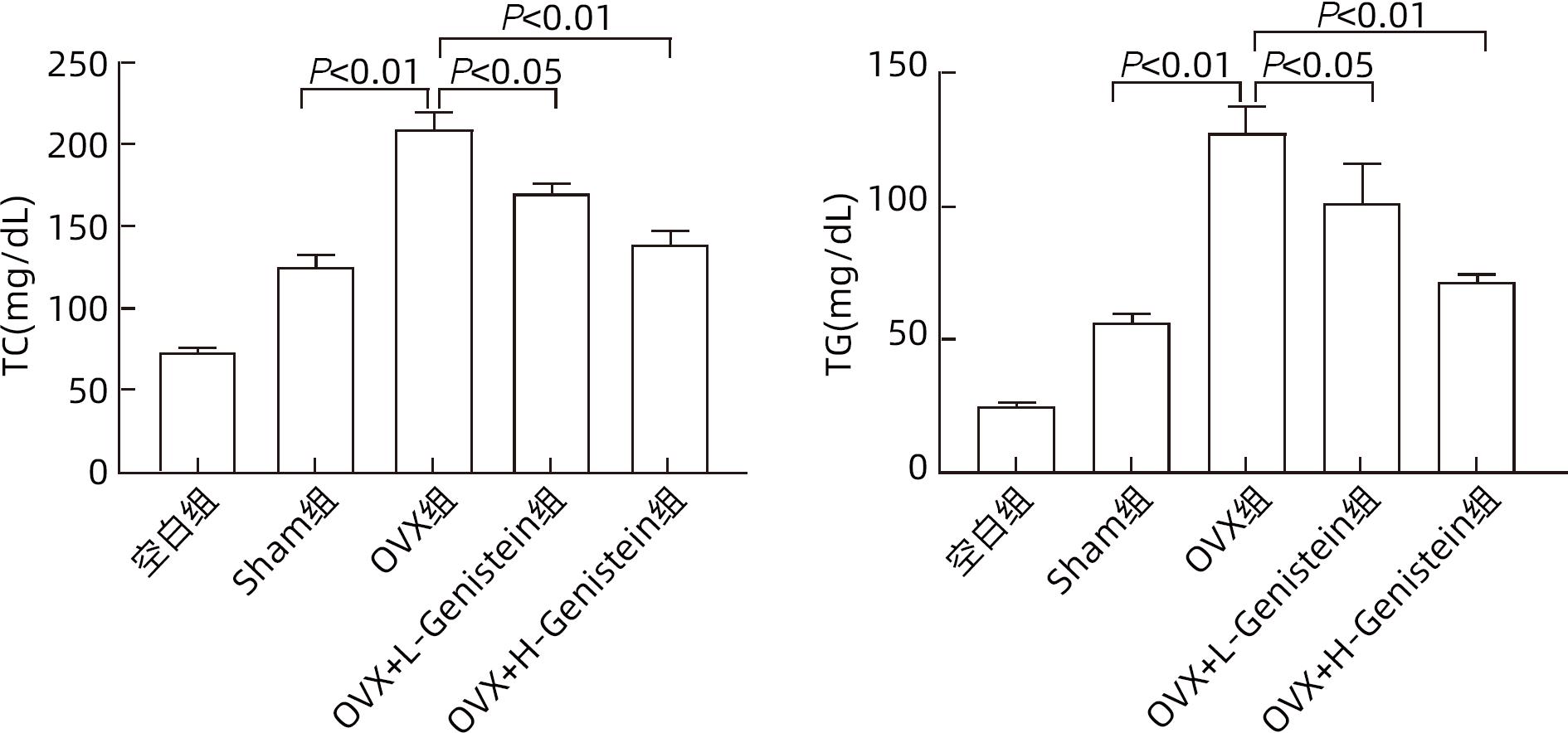
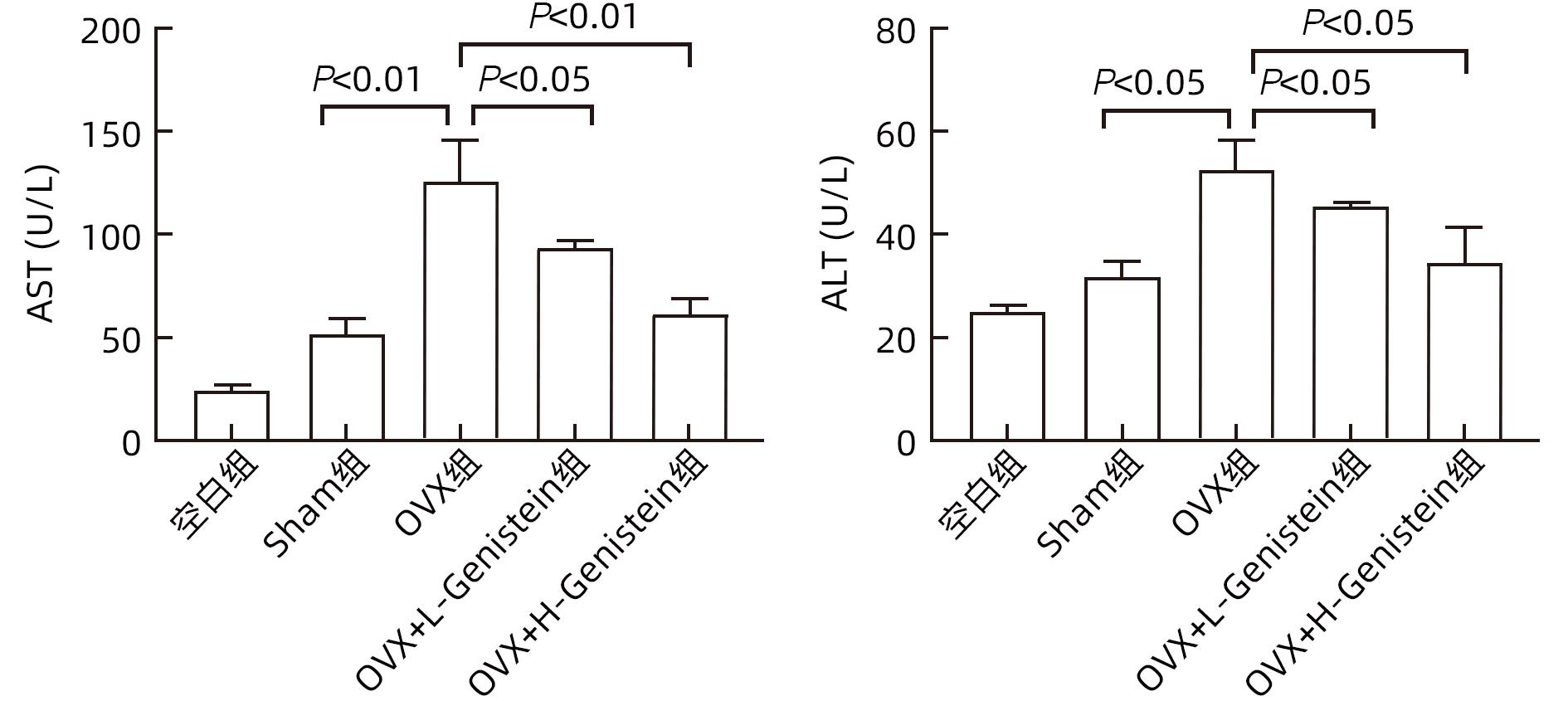
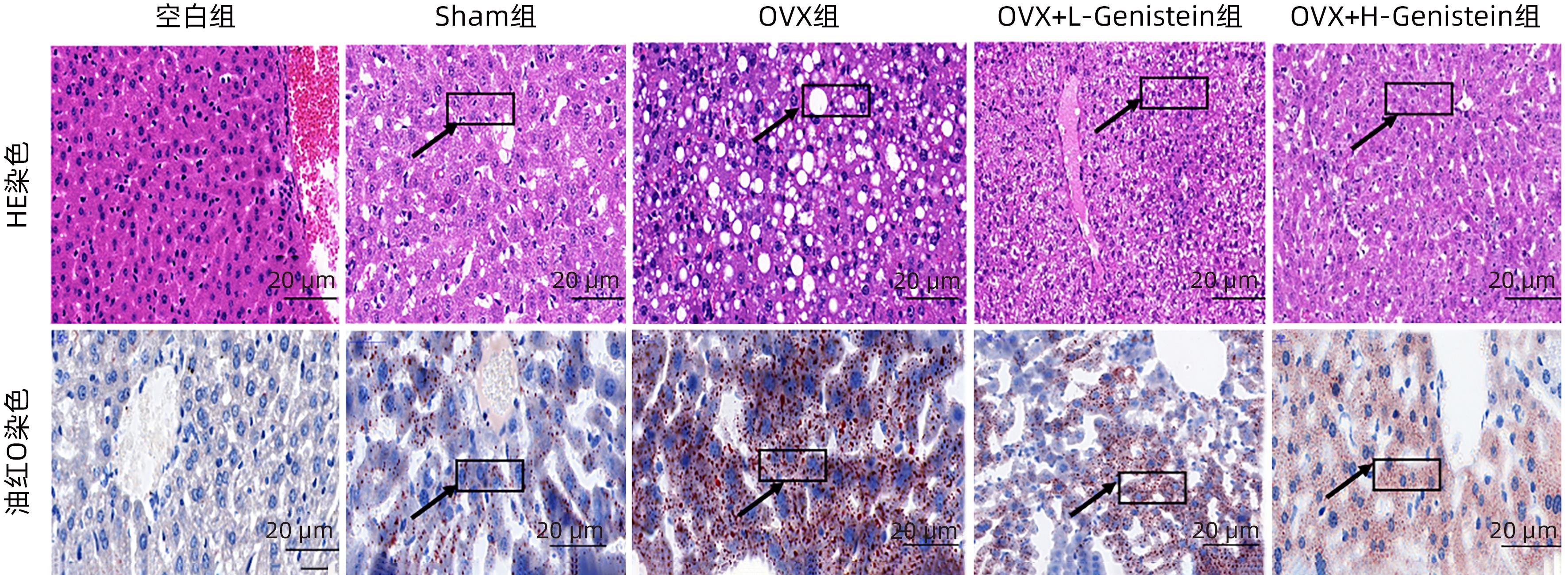
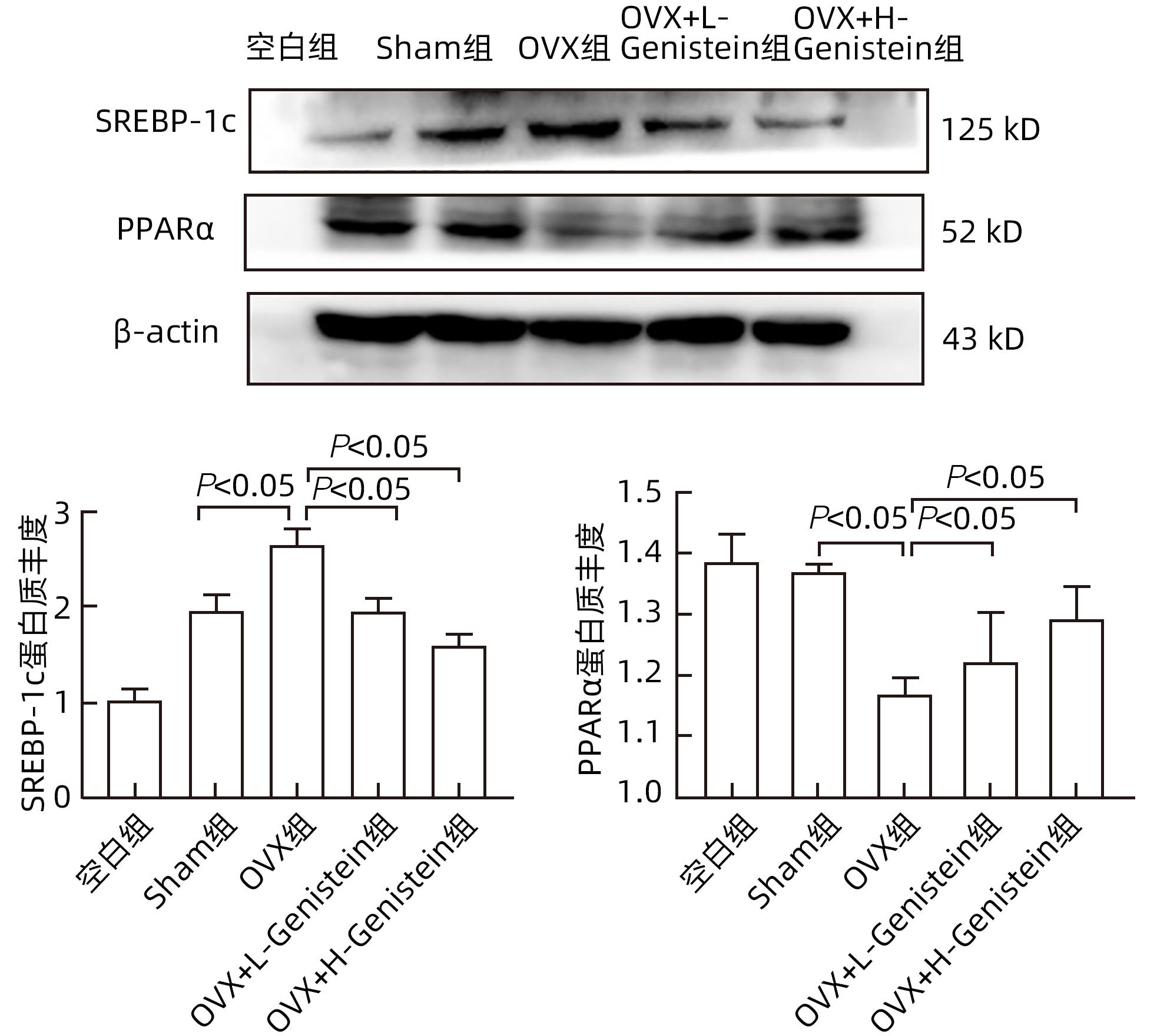






 DownLoad:
DownLoad: