| [1] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association of Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [2] |
CHEN MJ, LI X, TANG SH. Research progress in multi-dimensional evaluation of liver function in patients with liver failure[J]. Clin J Med Offic, 2023, 51( 9): 901- 903, 907. DOI: 10.16680/j.1671-3826.2023.09.05. |
| [3] |
CHOPYK DM, GRAKOUI A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159( 3): 849- 863. DOI: 10.1053/j.gastro.2020.04.077. |
| [4] |
QIANG R, LIU XZ, XU JC. The immune pathogenesis of acute-on-chronic liver failure and the danger hypothesis[J]. Front Immunol, 2022, 13: 935160. DOI: 10.3389/fimmu.2022.935160. |
| [5] |
GAN Y, LI XY, HAN SZ, et al. The cGAS/STING pathway: A novel target for cancer therapy[J]. Front Immunol, 2022, 12: 795401. DOI: 10.3389/fimmu.2021.795401. |
| [6] |
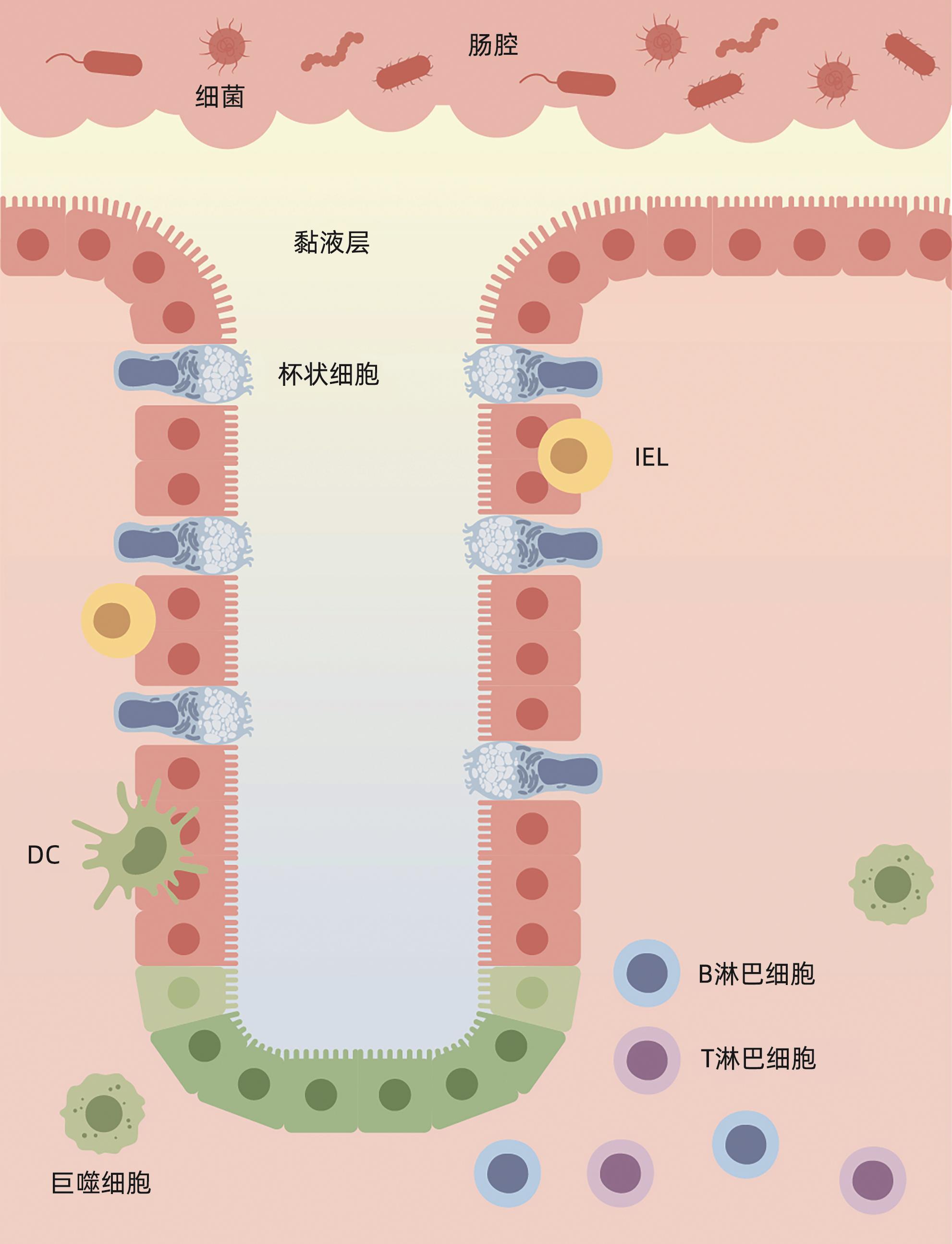
ALLAIRE JM, CROWLEY SM, LAW HT, et al. The intestinal epithelium: Central coordinator of mucosal immunity[J]. Trends Immunol, 2018, 39( 9): 677- 696. DOI: 10.1016/j.it.2018.04.002. |
| [7] |
DI TOMMASO N, GASBARRINI A, PONZIANI FR. Intestinal barrier in human health and disease[J]. Int J Environ Res Public Health, 2021, 18( 23): 12836. DOI: 10.3390/ijerph182312836. |
| [8] |
PAONE P, CANI PD. Mucus barrier, mucins and gut microbiota: The expected slimy partners?[J]. Gut, 2020, 69( 12): 2232- 2243. DOI: 10.1136/gutjnl-2020-322260. |
| [9] |
HENDRIKX T, SCHNABL B. Antimicrobial proteins: Intestinal guards to protect against liver disease[J]. J Gastroenterol, 2019, 54( 3): 209- 217. DOI: 10.1007/s00535-018-1521-8. |
| [10] |
LITVAK Y, MON KKZ, NGUYEN H, et al. Commensal enterobacteriaceae protect against Salmonella colonization through oxygen competition[J]. Cell Host Microbe, 2019, 25( 1): 128- 139. e 5. DOI: 10.1016/j.chom.2018.12.003. |
| [11] |
HIIPPALA K, JOUHTEN H, RONKAINEN A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation[J]. Nutrients, 2018, 10( 8): 988. DOI: 10.3390/nu10080988. |
| [12] |
VANCAMELBEKE M, VERMEIRE S. The intestinal barrier: A fundamental role in health and disease[J]. Expert Rev Gastroenterol Hepatol, 2017, 11( 9): 821- 834. DOI: 10.1080/17474124.2017.1343143. |
| [13] |
WEI Q, HUANG H. Insights into the role of cell-cell junctions in physiology and disease[J]. Int Rev Cell Mol Biol, 2013, 306: 187- 221. DOI: 10.1016/B978-0-12-407694-5.00005-5. |
| [14] |
MOWAT AM, AGACE WW. Regional specialization within the intestinal immune system[J]. Nat Rev Immunol, 2014, 14( 10): 667- 685. DOI: 10.1038/nri3738. |
| [15] |
DELFINI M, STAKENBORG N, VIOLA MF, et al. Macrophages in the gut: Masters in multitasking[J]. Immunity, 2022, 55( 9): 1530- 1548. DOI: 10.1016/j.immuni.2022.08.005. |
| [16] |
MARTÍNEZ-LÓPEZ M, IBORRA S, CONDE-GARROSA R, et al. Microbiota sensing by mincle-syk axis in dendritic cells regulates interleukin-17 and-22 production and promotes intestinal barrier integrity[J]. Immunity, 2019, 50( 2): 446- 461. e 9. DOI: 10.1016/j.immuni.2018.12.020. |
| [17] |
TEZUKA H, OHTEKI T. Regulation of IgA production by intestinal dendritic cells and related cells[J]. Front Immunol, 2019, 10: 1891. DOI: 10.3389/fimmu.2019.01891. |
| [18] |
SPENCER J, BEMARK M. Human intestinal B cells in inflammatory diseases[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 4): 254- 265. DOI: 10.1038/s41575-023-00755-6. |
| [19] |
MANN ER, LAM YK, UHLIG HH. Short-chain fatty acids: Linking diet, the microbiome and immunity[J]. Nat Rev Immunol, 2024, 24( 8): 577- 595. DOI: 10.1038/s41577-024-01014-8. |
| [20] |
LE N, MAZAHERY C, NGUYEN K, et al. Regulation of intestinal epithelial barrier and immune function by activated T cells[J]. Cell Mol Gastroenterol Hepatol, 2021, 11( 1): 55- 76. DOI: 10.1016/j.jcmgh.2020.07.004. |
| [21] |
YOO JS, OH SF. Unconventional immune cells in the gut mucosal barrier: Regulation by symbiotic microbiota[J]. Exp Mol Med, 2023, 55( 9): 1905- 1912. DOI: 10.1038/s12276-023-01088-9. |
| [22] |
GIL-CRUZ C, PEREZ-SHIBAYAMA C, ONDER L, et al. Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs[J]. Nat Immunol, 2016, 17( 12): 1388- 1396. DOI: 10.1038/ni.3566. |
| [23] |
HOU QH, HUANG JX, AYANSOLA H, et al. Intestinal stem cells and immune cell relationships: Potential therapeutic targets for inflammatory bowel diseases[J]. Front Immunol, 2021, 11: 623691. DOI: 10.3389/fimmu.2020.623691. |
| [24] |
SONNENBERG GF, MONTICELLI LA, ALENGHAT T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria[J]. Science, 2012, 336( 6086): 1321- 1325. DOI: 10.1126/science.1222551. |
| [25] |
LE BOURHIS L, MARTIN E, PÉGUILLET I, et al. Antimicrobial activity of mucosal-associated invariant T cells[J]. Nat Immunol, 2010, 11( 8): 701- 708. DOI: 10.1038/ni.1890. |
| [26] |
OLIVARES-VILLAGÓMEZ D, VAN KAER L. Intestinal intraepithelial lymphocytes: Sentinels of the mucosal barrier[J]. Trends Immunol, 2018, 39( 4): 264- 275. DOI: 10.1016/j.it.2017.11.003. |
| [27] |
KAYAMA H, OKUMURA R, TAKEDA K. Interaction between the microbiota, epithelia, and immune cells in the intestine[J]. Annu Rev Immunol, 2020, 38: 23- 48. DOI: 10.1146/annurev-immunol-070119-115104. |
| [28] |
CHEN BR, NI X, SUN R, et al. Commensal bacteria-dependent CD8αβ + T cells in the intestinal epithelium produce antimicrobial peptides[J]. Front Immunol, 2018, 9: 1065. DOI: 10.3389/fimmu.2018.01065. |
| [29] |
HOYTEMA VAN KONIJNENBURG DP, REIS BS, PEDICORD VA, et al. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection[J]. Cell, 2017, 171( 4): 783- 794. e 13. DOI: 10.1016/j.cell.2017.08.046. |
| [30] |
WELLS JM, BRUMMER RJ, DERRIEN M, et al. Homeostasis of the gut barrier and potential biomarkers[J]. Am J Physiol Gastrointest Liver Physiol, 2017, 312( 3): G171- G193. DOI: 10.1152/ajpgi.00048.2015. |
| [31] |
ZHANG B, DILIHUMAER ZYE, ZHANG SY, et al. Progress on pathogenesis and medical treatment of hepatitis B virus-related chronic and acute liver failure[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005. |
| [32] |
BIGGINS SW, ANGELI P, GARCIA-TSAO G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases[J]. Hepatology, 2021, 74( 2): 1014- 1048. DOI: 10.1002/hep.31884. |
| [33] |
KIM SE, PARK JW, KIM HS, et al. The role of gut dysbiosis in acute-on-chronic liver failure[J]. Int J Mol Sci, 2021, 22( 21): 11680. DOI: 10.3390/ijms222111680. |
| [34] |
BAJAJ JS, VARGAS HE, REDDY KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis[J]. Clin Gastroenterol Hepatol, 2019, 17( 4): 756- 765. e 3. DOI: 10.1016/j.cgh.2018.07.022. |
| [35] |
FERNÁNDEZ J, ACEVEDO J, WIEST R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis[J]. Gut, 2018, 67( 10): 1870- 1880. DOI: 10.1136/gutjnl-2017-314240. |
| [36] |
PHILIPS CA, AUGUSTINE P. Gut barrier and microbiota in cirrhosis[J]. J Clin Exp Hepatol, 2022, 12( 2): 625- 638. DOI: 10.1016/j.jceh.2021.08.027. |
| [37] |
FENG X, LIU DY, LI ZY, et al. Bioactive modulators targeting STING adaptor in cGAS-STING pathway[J]. Drug Discov Today, 2020, 25( 1): 230- 237. DOI: 10.1016/j.drudis.2019.11.007. |
| [38] |
BAI JL, LIU F. The cGAS-cGAMP-STING pathway: A molecular link between immunity and metabolism[J]. Diabetes, 2019, 68( 6): 1099- 1108. DOI: 10.2337/dbi18-0052. |
| [39] |
CHEN RH, DU JM, ZHU H, et al. The role of cGAS-STING signalling in liver diseases[J]. JHEP Rep, 2021, 3( 5): 100324. DOI: 10.1016/j.jhepr.2021.100324. |
| [40] |
LUTHER J, KHAN S, GALA MK, et al. Hepatic gap junctions amplify alcohol liver injury by propagating cGAS-mediated IRF3 activation[J]. Proc Natl Acad Sci USA, 2020, 117( 21): 11667- 11673. DOI: 10.1073/pnas.1911870117. |
| [41] |
ZHANG H. The role of autophagy and macrophage polarization mediated by STING pathway activation in the pathogenesis of HBV-related acute liver failure and the establishment of clinical prognosis model[D]. Hefei: Anhui Medical University, 2023.
张浩. STING通路活化介导自噬及巨噬细胞极化在HBV相关慢加急性肝衰竭发病机制的作用及临床预后模型的建立[D]. 合肥: 安徽医科大学, 2023.
|
| [42] |
YU T, CHENG HR, LI XL, et al. Design and synthesis of hederagenin derivatives modulating STING/NF-κB signaling for the relief of acute liver injury in septic mice[J]. Eur J Med Chem, 2023, 245( Pt 1): 114911. DOI: 10.1016/j.ejmech.2022.114911. |
| [43] |
CANESSO MCC, LEMOS L, NEVES TC, et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation[J]. Mucosal Immunol, 2018, 11( 3): 820- 834. DOI: 10.1038/mi.2017.88. |
| [44] |
LOUIE A, BHANDULA V, PORTNOY DA. Secretion of c-di-AMP by Listeria monocytogenes leads to a STING-dependent antibacterial response during enterocolitis[J]. Infect Immun, 2020, 88( 12): e00407-20. DOI: 10.1128/IAI.00407-20. |
| [45] |
ZHANG Q, CHEN QY, YAN CS, et al. The absence of STING ameliorates non-alcoholic fatty liver disease and reforms gut bacterial community[J]. Front Immunol, 2022, 13: 931176. DOI: 10.3389/fimmu.2022.931176. |
| [46] |
ZHANG XF, WU J, LIU QJ, et al. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion[J]. Cell Death Dis, 2020, 11( 12): 1050. DOI: 10.1038/s41419-020-03239-6. |
| [47] |
AL-SADI R, GUO SH, YE DM, et al. TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-κB pathway[J]. Am J Pathol, 2016, 186( 5): 1151- 1165. DOI: 10.1016/j.ajpath.2015.12.016. |
| [48] |
HU QY, REN HJ, LI GW, et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis[J]. EBioMedicine, 2019, 41: 497- 508. DOI: 10.1016/j.ebiom.2019.02.055. |
| [49] |
MARTIN GR, BLOMQUIST CM, HENARE KL, et al. Stimulator of interferon genes(STING) activation exacerbates experimental colitis in mice[J]. Sci Rep, 2019, 9( 1): 14281. DOI: 10.1038/s41598-019-50656-5. |
| [50] |
SCHAUPP L, MUTH S, ROGELL L, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells[J]. Cell, 2020, 181( 5): 1080- 1096. e 19. DOI: 10.1016/j.cell.2020.04.022. |
| [51] |
GUTIERREZ-MERINO J, ISLA B, COMBES T, et al. Beneficial bacteria activate type-I interferon production via the intracellular cytosolic sensors STING and MAVS[J]. Gut Microbes, 2020, 11( 4): 771- 788. DOI: 10.1080/19490976.2019.1707015. |







 DownLoad:
DownLoad: