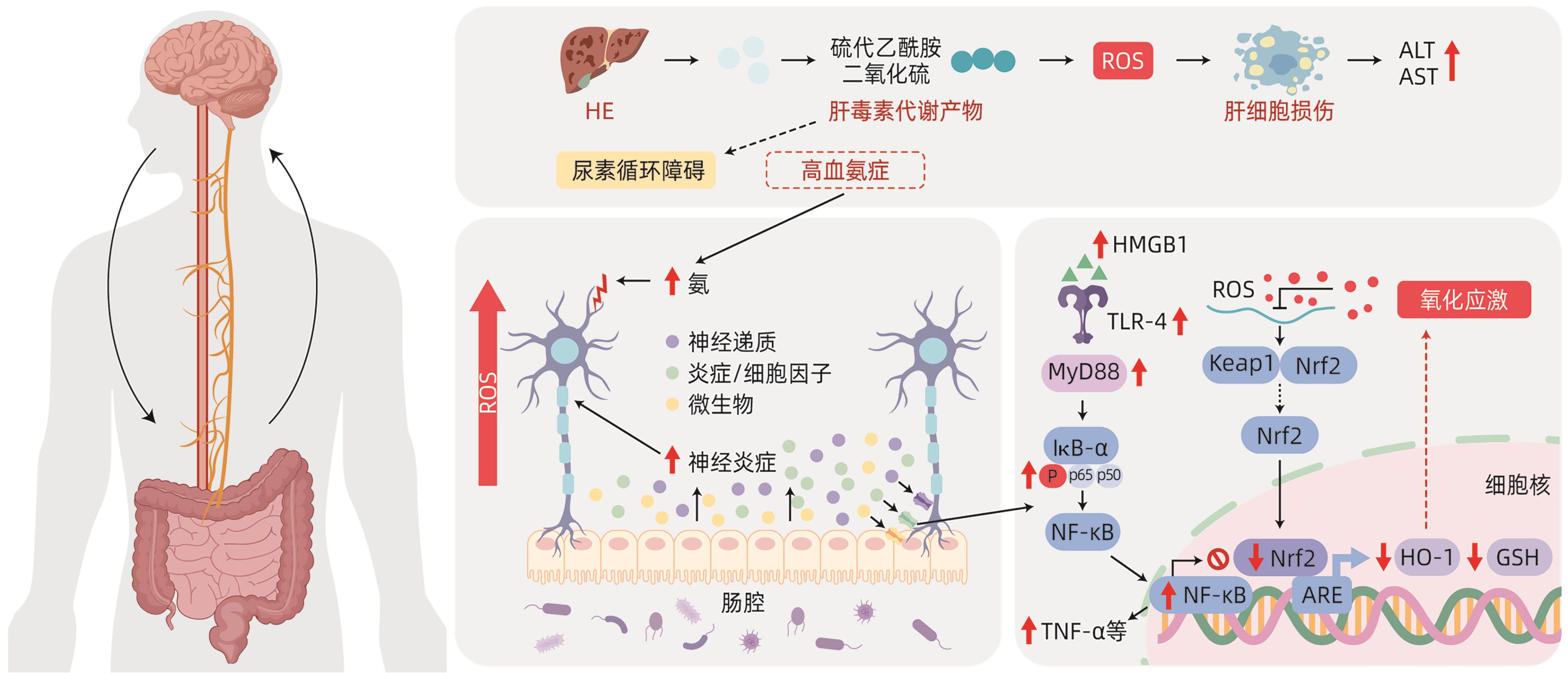
| [1] |
RUDLER M, WEISS N, BOUZBIB C, et al. Diagnosis and management of hepatic encephalopathy[J]. Clin Liver Dis, 2021, 25( 2): 393- 417. DOI: 10.1016/j.cld.2021.01.008. |
| [2] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [3] |
ELSAID MI, RUSTGI VK. Epidemiology of hepatic encephalopathy[J]. Clin Liver Dis, 2020, 24( 2): 157- 174. DOI: 10.1016/j.cld.2020.01.001. |
| [4] |
LIN Y, YAN GJ, FENG F, et al. Association between cholesterol and liver regeneration and its significance and potential value in clinical treatment of liver failure[J]. J Clin Hepatol, 2022, 38( 3): 708- 713. DOI: 10.3969/j.issn.1001-5256.2022.03.044. |
| [5] |
DU YQ, WANG M, HUANG GC, et al. Therapeutic effect of retention enema with compound rhubarb decoction on a rat model of minimal hepatic encephalopathy based on bile acid metabolomics[J]. J Clin Hepatol, 2023, 39( 10): 2348- 2357. DOI: 10.3969/j.issn.1001-5256.2023.10.012. |
| [6] |
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of hepatic encephalopathy[J]. J Hepatol, 2022, 77( 3): 807- 824. DOI: 10.1016/j.jhep.2022.06.001. |
| [7] |
PUN CK, HUANG HC, CHANG CC, et al. Hepatic encephalopathy: From novel pathogenesis mechanism to emerging treatments[J]. J Chin Med Assoc, 2023, 87( 3): 245- 251. DOI: 10.1097/JCMA.0000000000001041. |
| [8] |
RONALDSON PT, DAVIS TP. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity[J]. J Cereb Blood Flow Metab, 2020, 40( 1_suppl): S6- S24. DOI: 10.1177/0271678X20951995. |
| [9] |
OCHOA-SANCHEZ R, TAMNANLOO F, ROSE CF. Hepatic encephalopathy: From metabolic to neurodegenerative[J]. Neurochem Res, 2021, 46( 10): 2612- 2625. DOI: 10.1007/s11064-021-03372-4. |
| [10] |
|
| [11] |
AGIRMAN G, YU KB, HSIAO EY. Signaling inflammation across the gut-brain axis[J]. Science, 2021, 374( 6571): 1087- 1092. DOI: 10.1126/science.abi6087. |
| [12] |
VIDAL-CEVALLOS P, CHÁVEZ-TAPIA NC, URIBE M. Current approaches to hepatic encephalopathy[J]. Ann Hepatol, 2022, 27( 6): 100757. DOI: 10.1016/j.aohep.2022.100757. |
| [13] |
ORZEŁ-GAJOWIK K, MILEWSKI K, ZIELIŃSKA M. miRNA-ome plasma analysis unveils changes in blood-brain barrier integrity associated with acute liver failure in rats[J]. Fluids Barriers CNS, 2023, 20( 1): 92. DOI: 10.1186/s12987-023-00484-7. |
| [14] |
SCOTT SA, FU JJ, CHANG PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor[J]. Proc Natl Acad Sci U S A, 2020, 117( 32): 19376- 19387. DOI: 10.1073/pnas.2000047117. |
| [15] |
KHALIL HMA, ELIWA HA, EL-SHIEKH RA, et al. Ashwagandha(Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways[J]. J Ethnopharmacol, 2021, 277: 114141. DOI: 10.1016/j.jep.2021.114141. |
| [16] |
ALI SA, DATUSALIA AK. Protective effects of Tinospora cordifolia miers extract against hepatic and neurobehavioral deficits in thioacetamide-induced hepatic encephalopathy in rats via modulating hyperammonemia and glial cell activation[J]. J Ethnopharmacol, 2024, 323: 117700. DOI: 10.1016/j.jep.2023.117700. |
| [17] |
CRYAN JF, O’RIORDAN KJ, COWAN CSM, et al. The microbiota-gut-brain axis[J]. Physiol Rev, 2019, 99( 4): 1877- 2013. DOI: 10.1152/physrev.00018.2018. |
| [18] |
XU CL, LEE SK, ZHANG DC, et al. The gut microbiome regulates psychological-stress-induced inflammation[J]. Immunity, 2020, 53( 2): 417- 428. DOI: 10.1016/j.immuni.2020.06.025. |
| [19] |
LOUVEAU A, HERZ J, ALME MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature[J]. Nat Neurosci, 2018, 21( 10): 1380- 1391. DOI: 10.1038/s41593-018-0227-9. |
| [20] |
JAMESON KG, OLSON CA, KAZMI SA, et al. Toward understanding microbiome-neuronal signaling[J]. Mol Cell, 2020, 78( 4): 577- 583. DOI: 10.1016/j.molcel.2020.03.006. |
| [21] |
JIA W, RAJANI C, KADDURAH-DAOUK R, et al. Expert insights: The potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer’s disease and hepatic encephalopathy[J]. Med Res Rev, 2020, 40( 4): 1496- 1507. DOI: 10.1002/med.21653. |
| [22] |
LIU T, ZHANG LY, JOO D, et al. NF-κB signaling in inflammation[J]. Signal Transduct Target Ther, 2017, 2: 17023. DOI: 10.1038/sigtrans.2017.23. |
| [23] |
ESSAM RM, SAADAWY MA, GAMAL M, et al. Lactoferrin averts neurological and behavioral impairments of thioacetamide-induced hepatic encephalopathy in rats via modulating HGMB1/TLR-4/MyD88/Nrf2 pathway[J]. Neuropharmacology, 2023, 236: 109575. DOI: 10.1016/j.neuropharm.2023.109575. |
| [24] |
KIGERL KA, LAI WM, WALLACE LM, et al. High mobility group box-1(HMGB1) is increased in injured mouse spinal cord and can elicit neurotoxic inflammation[J]. Brain Behav Immun, 2018, 72: 22- 33. DOI: 10.1016/j.bbi.2017.11.018. |
| [25] |
YOU XH, WANG LX, FENG DC. Relationship of the intestinal flora and irritable bowel syndrome sub-typing with the functional changes in the neuroendocrine axis[J]. J Clin Exp Med, 2022, 21( 15): 1667- 1670. DOI: 10.3969/j.issn.1671-4695.2022.15.027. |
| [26] |
PENNY HA, DOMINGUES RG, KRAUSS MZ, et al. Rhythmicity of intestinal IgA responses confers oscillatory commensal microbiota mutualism[J]. Sci Immunol, 2022, 7( 75): eabk2541. DOI: 10.1126/sciimmunol.abk2541. |
| [27] |
ROJAS OL, PRÖBSTEL AK, PORFILIO EA, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10[J]. Cell, 2019, 176( 3): 610- 624. DOI: 10.1016/j.cell.2018.11.035. |
| [28] |
BONAZ B, BAZIN T, PELLISSIER S. The vagus nerve at the interface of the microbiota-gut-brain axis[J]. Front Neurosci, 2018, 12: 49. DOI: 10.3389/fnins.2018.00049. |
| [29] |
BLUTHÉ RM, MICHAUD B, KELLEY KW, et al. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes[J]. Neuroreport, 1996, 7( 15-17): 2823- 2827. DOI: 10.1097/00001756-199611040-00083. |
| [30] |
MCVEY NEUFELD KA, BIENENSTOCK J, BHARWANI A, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling[J]. Sci Rep, 2019, 9( 1): 14290. DOI: 10.1038/s41598-019-50807-8. |
| [31] |
MORAIS LH, SCHREIBER HL 4th. MAZMANIAN SK The gut microbiotabrain axis in behaviour and brain disorders[J]. Nat Rev Microbiol, 2021, 19( 4): 241- 255. DOI: 10.1038/s41579-020-00460-0. |
| [32] |
MARTÍNEZ-LOZADA Z, ORTEGA A. Glutamatergic transmission: A matter of three[J]. Neural Plast, 2015, 2015: 787396. DOI: 10.1155/2015/787396. |
| [33] |
CABRERA-PASTOR A, LLANSOLA M, MONTOLIU C, et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications[J]. Acta Physiol(Oxf), 2019, 226( 2): e13270. DOI: 10.1111/apha.13270. |
| [34] |
KOSENKOV AM, GAIDIN SG, SERGEEV AI, et al. Fast changes of NMDA and AMPA receptor activity under acute hyperammonemia in vitro[J]. Neurosci Lett, 2018, 686: 80- 86. DOI: 10.1016/j.neulet.2018.08.054. |
| [35] |
CAULI O, GONZÁLEZ-USANO A, CABRERA-PASTOR A, et al. Blocking NMDA receptors delays death in rats with acute liver failure by dual protective mechanisms in kidney and brain[J]. NeuroMolecular Med, 2014, 16( 2): 360- 375. DOI: 10.1007/s12017-013-8283-5. |
| [36] |
KULLMANN DM, RUIZ A, RUSAKOV DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: Where and why?[J]. Prog Biophys Mol Biol, 2005, 87( 1): 33- 46. DOI: 10.1016/j.pbiomolbio.2004.06.003. |
| [37] |
FRIED DE, WATSON RE, ROBSON SC, et al. Ammonia modifies enteric neuromuscular transmission through glial γ-aminobutyric acid signaling[J]. Am J Physiol Gastrointest Liver Physiol, 2017, 313( 6): G570- G580. DOI: 10.1152/ajpgi.00154.2017. |
| [38] |
MA FH, YANG L, SUN ZR, et al. Neurotransmitter-derived lipidoids(NT-lipidoids) for enhanced brain delivery through intravenous injection[J]. Sci Adv, 2020, 6( 30): eabb4429. DOI: 10.1126/sciadv.abb4429. |
| [39] |
TERSTAPPEN GC, MEYER AH, BELL RD, et al. Strategies for delivering therapeutics across the blood-brain barrier[J]. Nat Rev Drug Discov, 2021, 20( 5): 362- 383. DOI: 10.1038/s41573-021-00139-y. |



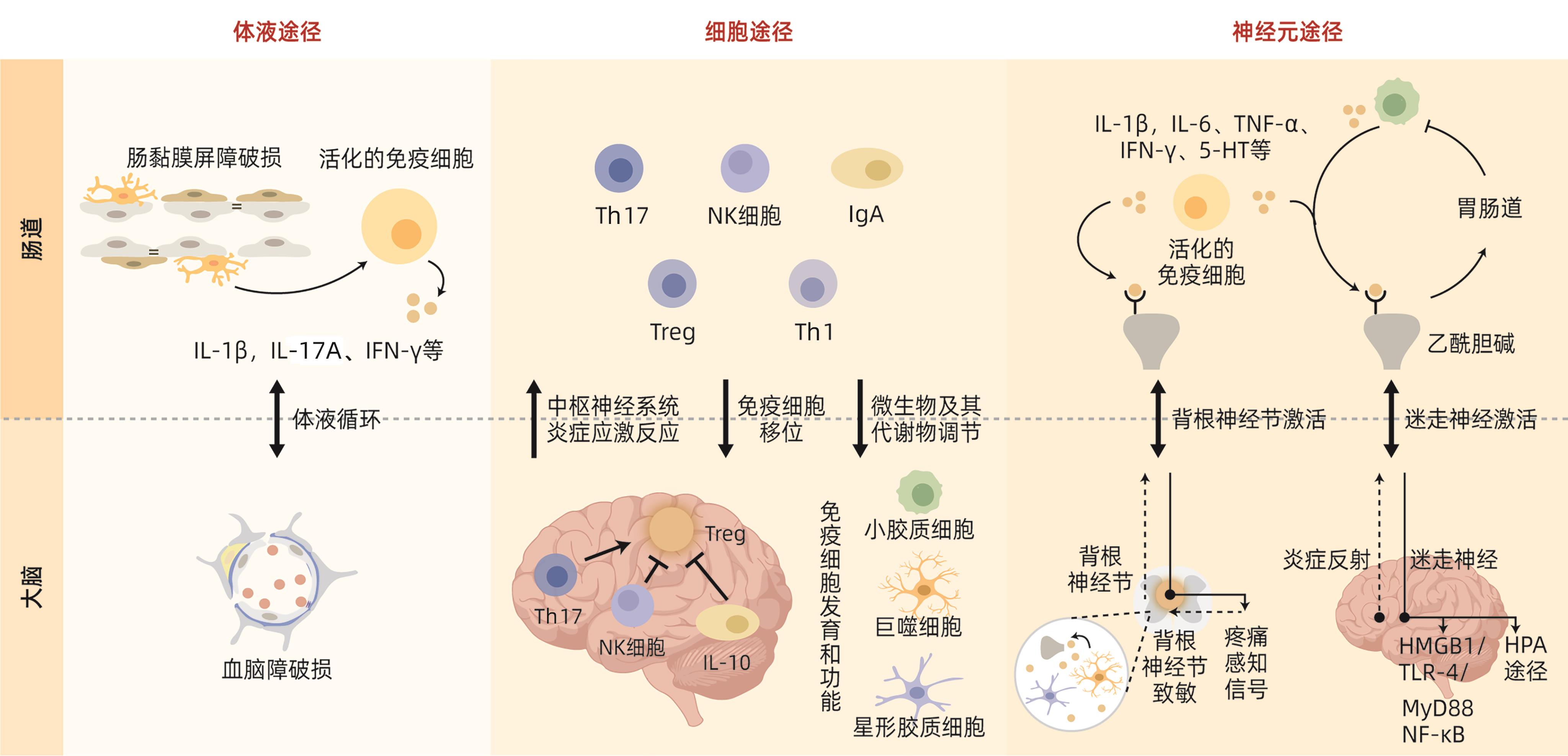




 DownLoad:
DownLoad: