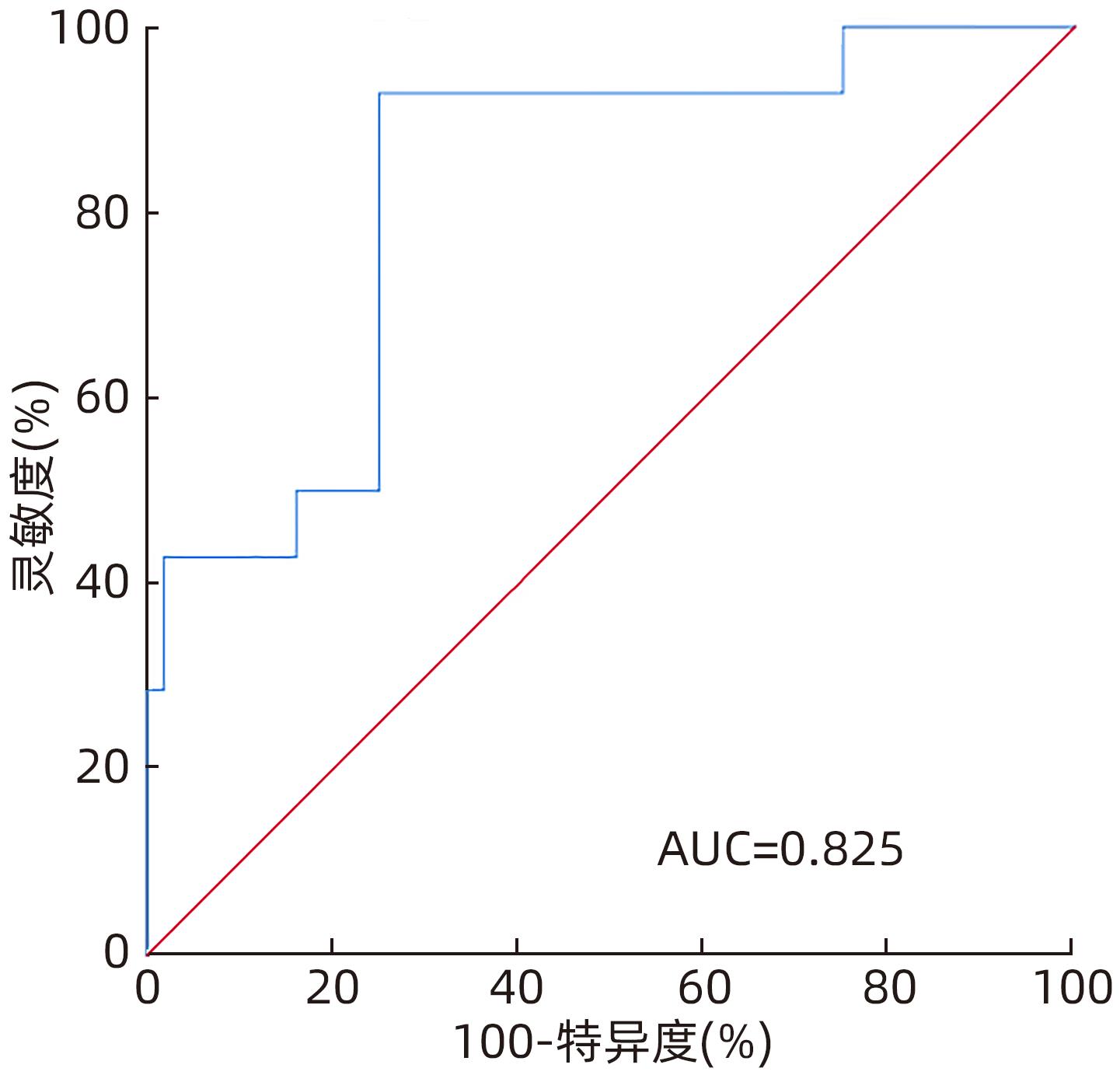
| [1] |
RAHBARI NN, GARDEN OJ, PADBURY R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery(ISGLS)[J]. Surgery, 2011, 149( 5): 713- 724. DOI: 10.1016/j.surg.2010.10.001. |
| [2] |
HAEGELE S, REITER S, WANEK D, et al. Perioperative non-invasive indocyanine green-clearance testing to predict postoperative outcome after liver resection[J]. PLoS One, 2016, 11( 11): e0165481. DOI: 10.1371/journal.pone.0165481. |
| [3] |
DU ZG, LI B, FENG X, et al. Combined indocyanine green test and standard remnant liver volume to predict post-hepatectomy hepatic insufficiency for the patients with hepatocellular carcinoma[J]. Chin J Surg, 2010, 48( 3): 189- 192. DOI: 10.3760/cma.j.issn.0529-5815.2010.03.010. |
| [4] |
DONG JH, ZHENG SS, CHEN XP, et al. Consensus on evaluation of hepatic functional reserve before hepatectomy(2011 edition)[J]. Chin J Dig Surg, 2011, 10( 1): 20- 25. DOI: 10.3760/cma.j.issn.1673-9752.2011.01.006. |
| [5] |
XU LN, XU YY, GAO DW. Impact of operative and peri-operative factors on the long-term prognosis of primary liver cancer patients undergoing hepatectomy[J]. J Huazhong Univ Sci Technolog Med Sci, 2016, 36( 4): 523- 528. DOI: 10.1007/s11596-016-1619-2. |
| [6] |
TAKAHASHI K, KUROKAWA T, OSHIRO Y, et al. Postoperative decrease in platelet counts is associated with delayed liver function recovery and complications after partial hepatectomy[J]. Tohoku J Exp Med, 2016, 239( 1): 47- 55. DOI: 10.1620/tjem.239.47. |
| [7] |
LIU Y, CHEN ZL, YU XX, et al. Risk factors for hepatic insufficiency after major hepatectomy in non-cirrhotic patients[J]. Asian J Surg, 2021, 44( 10): 1324- 1325. DOI: 10.1016/j.asjsur.2021.06.046. |
| [8] |
APERS T, HENDRIKX B, BRACKE B, et al. Parenchymal-sparing hepatectomy with hepatic vein resection and reconstruction[J]. Acta Chir Belg, 2022, 122( 5): 334- 340. DOI: 10.1080/00015458.2021.1915021. |
| [9] |
SØREIDE JA, DESHPANDE R. Post hepatectomy liver failure(PHLF)-Recent advances in prevention and clinical management[J]. Eur J Surg Oncol, 2021, 47( 2): 216- 224. DOI: 10.1016/j.ejso.2020.09.001. |
| [10] |
HAYASHI H, BEPPU T, OKABE H, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy[J]. Surgery, 2015, 157( 1): 20- 26. DOI: 10.1016/j.surg.2014.06.013. |
| [11] |
MOON YJ, KIM SH, KIM JW, et al. Comparison of postoperative coagulation profiles and outcome for sugammadex versus pyridostigmine in 992 living donors after living-donor hepatectomy[J]. Medicine, 2018, 97( 11): e0129. DOI: 10.1097/MD.0000000000010129. |
| [12] |
DE RUDDER M, DILI A, STÄRKEL P, et al. Critical role of LSEC in post-hepatectomy liver regeneration and failure[J]. Int J Mol Sci, 2021, 22( 15): 8053. DOI: 10.3390/ijms22158053. |
| [13] |
ZHANG ZM, OUYANG GX, WANG P, et al. Safe standard remnant liver volume after hepatectomy in HCC patients in different stages of hepatic fibrosis[J]. BMC Surg, 2021, 21( 1): 57. DOI: 10.1186/s12893-021-01065-x. |
| [14] |
YOSHIDA M, SHIRAISHI S, SAKAMOTO F, et al. Assessment of hepatic functional regeneration after hepatectomy using(99m)Tc-GSA SPECT/CT fused imaging[J]. Ann Nucl Med, 2014, 28( 8): 780- 788. DOI: 10.1007/s12149-014-0872-3. |
| [15] |
FUNG J, POON RTP, YU WC, et al. Use of liver stiffness measurement for liver resection surgery: Correlation with indocyanine green clearance testing and post-operative outcome[J]. PLoS One, 2013, 8( 8): e72306. DOI: 10.1371/journal.pone.0072306. |
| [16] |
CHEN ZS, LIN KC, LIU JF. Application of three-dimensional visualization in surgical operation for primary liver cancer[J]. J Clin Hepatol, 2022, 38( 3): 505- 509. DOI: 10.3969/j.issn.1001-5256.2022.03.003. |
| [17] |
BOWEN SR, CHAPMAN TR, BORGMAN J, et al. Measuring total liver function on sulfur colloid SPECT/CT for improved risk stratification and outcome prediction of hepatocellular carcinoma patients[J]. EJNMMI Res, 2016, 6( 1): 57. DOI: 10.1186/s13550-016-0212-9. |
| [18] |
ETRA JW, SQUIRES MH 3rd, FISHER SB, et al. Early identification of patients at increased risk for hepatic insufficiency, complications and mortality after major hepatectomy[J]. HPB, 2014, 16( 10): 875- 883. DOI: 10.1111/hpb.12270. |
| [19] |
GAO YY, ZHANG X, LI FH, et al. Measurement of glycosylated albumin and its application value in liver cirrhosis patients with different Child-Pugh classes[J]. J Clin Hepatol, 2022, 38( 2): 347- 351. DOI: 10.3969/j.issn.1001-5256.2022.02.018. |
| [20] |
ZHAO D, YE JD, LI HL, et al. Application of liver three-dimensional visualized reconstruction technique in hepatectomy for children with compli-cated hepatoblastoma[J]. J Clin Hepatol, 2021, 37( 9): 2130- 2135. DOI: 10.3969/j.issn.1001-5256.2021.09.025. |



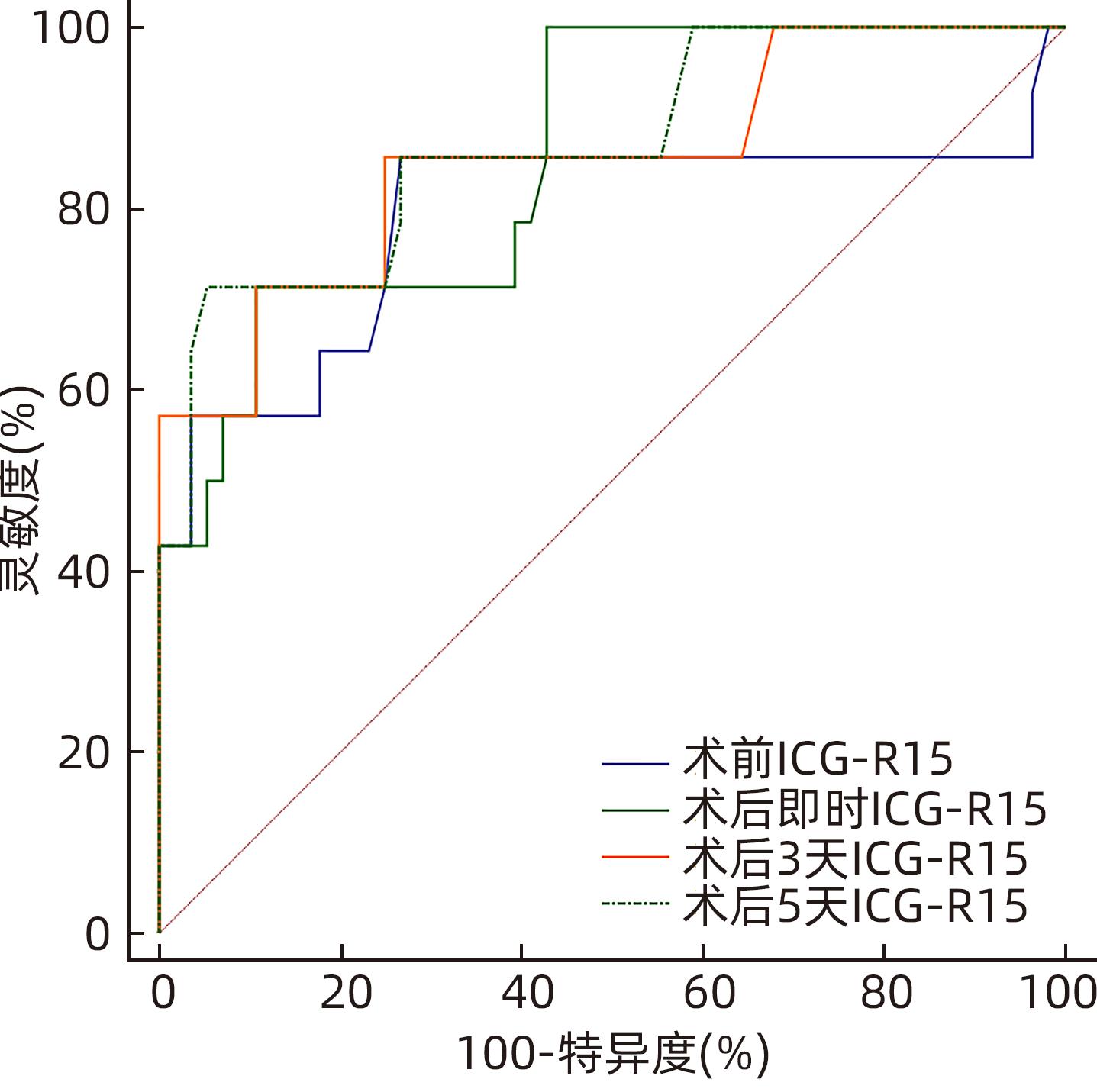




 DownLoad:
DownLoad: