差异表达microRNA参与刀豆蛋白A诱导的自身免疫性肝炎小鼠模型肝损伤的功能分析
DOI: 10.3969/j.issn.1001-5256.2021.06.028
A functional analysis of differentially expressed microRNAs involved in liver injury in mice with autoimmune hepatitis induced by concanavalin A
-
摘要:
目的 探讨了刀豆蛋白A(ConA)诱导的自身免疫性肝炎(AIH)小鼠模型肝损伤发生发展过程中差异表达microRNA(miRNA)的变化和潜在作用。 方法 健康雄性SPF级C57BL/6小鼠8只,随机分为模型组(n=4)和对照组(n=4)。模型组按15 mg/kg的剂量给予尾静脉注射ConA,对照组给予等剂量生理盐水,造模8 h后处死全部小鼠,提取肝组织总RNA,采用基因芯片筛选差异表达miRNA,并对表达上调及表达下调的miRNA进行靶基因预测和功能分析。两组间比较采用独立样本t检验鉴定差异表达的miRNA。 结果 主成分分析结果显示,基因芯片中所提取数据的组间差异满足进一步分析的条件。与对照组比较,模型组小鼠肝脏中共检测到31个上调和18个下调表达的miRNA,与959个(601个上调,358个下调)靶基因存在调控关系。GO分析显示,与对照组比较,模型组中表达上调miRNA的靶基因主要具备"DNA binding"(P值=1.47×10-6)等分子功能,参与"transcription,DNA-templated"(P=2.36×10-7) 等生物学过程,并主要富集在"neuronal cell body"(P=5.99×10-6)等细胞组分。表达下调miRNA的靶基因主要具备"RNA polymerase Ⅱ proximal promoter sequence-specific DNA binding"(P=2.49×10-6)等分子功能,参与"regulation of transcription, DNA-templated"(P=1.64×10-11)等生物过程,并主要富集在"nucleoplasm"(P=4.30×10-10)等细胞组分。KEGG通路分析结果表明上调靶点主要涉及"Endocytosis"(P=0.000 4)等,下调靶点主要涉及"Hippo信号通路"(P=0.004)。 结论 AIH的发病中存在miRNA差异表达,这些miRNA差异表达谱可以为AIH的临床治疗提供新的靶点。 Abstract:Objective To investigate the changes and potential effects of differentially expressed microRNAs (miRNAs) in the development and progression of liver injury in a mouse model of autoimmune hepatitis (AIH) induced by concanavalin A (ConA). Methods Eight healthy male specific pathogen-free C57BL/6 mice were randomly divided into model group and control group, with four mice in each group. The mice in the model group were given tail vein injection of ConA 15 mg/kg, and those in the control group were given an equal volume of normal saline. All mice were sacrificed after 8 hours of modeling, Total RNA in liver tissue was extracted, gene microarray was used to screen out differentially expressed miRNAs, and target prediction and function analysis were performed for upregulated and downregulated miRNAs. The independent samples t-test was used for comparison of differentially expressed miRNAs between two groups. Results The principal component analysis showed that the inter-group difference of the data extracted by gene microarray met the conditions for further analysis. Compared with the control group, the model group had 31 upregulated miRNAs and 18 downregulated miRNAs in mouse liver, which had a regulatory relationship with 959 target genes (601 upregulated genes and 358 downregulated genes). GO analysis showed that in the model group, the target genes of the upregulated miRNAs mainly had the molecular functions such as "DNA binding" (P=1.47×10-6), participated in the biological processes such as "transcription, DNA-templated" (P=2.36×10-7), and were mainly enriched in the cellular components such as "neuronal cell body" (P=5.99×10-6), while the target genes of the downregulated miRNAs had the molecular functions such as "RNA polymerase Ⅱ proximal promoter sequence-specific DNA binding" (P=2.49×10-6), participated in the biological processes such as "regulation of transcription, DNA-templated" (P=1.64×10-11), and were mainly enriched in the cellular components such as "nucleoplasm" (P=4.30×10-10). KEGG pathway enrichment analysis showed that the target genes of the upregulated miRNAs were mainly enriched in "Endocytosis" (P=0.000 4), while the target genes of the downregulated miRNAs were mainly enriched in the "Hippo signaling pathway" (P=0.004), and the above functional analysis results were statistically significant (P < 0.05). Conclusion There are differentially expressed miRNAs in the pathogenesis of AIH, and these differentially expressed miRNAs can provide new targets for the clinical treatment of AIH. -
Key words:
- Hepatitis, Autoimmune /
- MicroRNAs /
- Computational Biology
-
自身免疫性肝炎(autoimmune hepatitis,AIH)是一种由肝细胞免疫介导的炎症疾病,其特征是肝实质进行性破坏,如果治疗不及时,后期会导致肝硬化、肝衰竭甚至死亡[1]。该病多发于欧美发达国家的女性当中,其发病率和流行率在当前世界范围内正在不断增加[2]。AIH的确切病因及发病机制目前尚不完全明确,缺少特异性的研究靶点,AIH的诊断和治疗仍面临巨大挑战[2-3]。microRNA(miRNA)作为一种高度组织特异性的生物标志物,在肝脏炎症或自身免疫性疾病中具有重要的调节潜力[4]。目前有关AIH中miRNA差异表达谱的研究未见报道。因此本研究拟采用微阵列芯片对刀豆蛋白A(concanavalin A,ConA)诱导肝损伤小鼠模型的差异表达miRNA进行筛选,通过生物信息学方法对miRNA的靶基因进行预测和功能分析,以期探讨miRNA在AIH发病中的潜在作用,为临床治疗AIH提供新的靶点。
1. 材料和方法
1.1 实验动物
雄性SPF级C57BL/6小鼠(鼠龄6周)8只,体质量为(20±2) g,购自北京维通利华实验动物技术有限公司[生产许可证编号: SCXK (京) 2016-0006,使用许可证编号: SYXK (京) 2017-0022]。小鼠常规饲养,自由摄食。
1.2 主要实验试剂
ConA购自索莱宝科技有限公司(北京),Maxima逆转录酶购自赛默飞世尔科技(上海),水合氯醛、Trizol试剂和UNlQ-10柱状总RNA分离试剂盒购自生工生物工程股份有限公司(上海)。qRT-PCR引物序列由生工生物工程股份有限公司合成。
1.3 实验方法
将小鼠随机分为模型组和对照组,每组4只。模型组按15 mg/kg的剂量给予尾静脉注射ConA[5-6],对照组给予等剂量生理盐水,造模8 h后处死全部小鼠。低温无菌条件下取出肝组织,用于后续进行肝脏差异表达基因筛选和数据分析。
1.4 微阵列杂交和数据分析
提取小鼠肝组织总RNA,利用NanoDrop超微量分光光度计(ND-2000,中国上海赛默飞世尔科技有限公司)定量,用生物分析仪(2100,美国安捷伦科技公司)检测RNA完整性。RNA质检合格后,根据小鼠miRNA微阵列芯片(070155,美国安捷伦科技公司)说明书按顺序进行样品标记、微阵列杂交和洗涤等实验操作。清洗完毕,使用扫描仪对阵列进行扫描得到原始图像,通过Feature Extraction 10.7.1.1软件对原始数据进行扫描分析,并从微阵列芯片图像中提取数据。接着对原始数据进行quantile标准化,过滤标准化后的数据,在用于比较的每组样本中至少有一组75%标记为"Detected"的探针留下,以进行后续分析。通过主成分分析(principal component analysis,PCA)验证实验数据的可信度;利用t检验的P值和倍数变化值(fold change, FC)进行差异基因筛选,并且以上调或者下调倍数变化值|FC|≥2.0和P≤0.05为筛选的标准阈值。
1.5 miRNA靶基因预测及GO富集、KEGG富集通路分析
通过miRWalk在线数据库(mirwalk.umm.uni-heidelberg.de/和miRDB在线数据库(www.mirdb.org/对所得到差异表达miRNA的靶基因进行预测,并根据miRDB(预测得分>80分)和miRWalk(P=1)的条件取交集,以最终确定差异表达miRNA的靶基因。对所得到的表达上调和表达下调miRNA的靶基因进行GO分析,包括生物学过程(biological process,BP)、分子功能(molecular function,MF)以及细胞组分(cell component,CC)和KEGG通路富集分析。以错误检出率(false discovery rate,FDR)≤0.05及P≤0.05作为筛选有统计学意义的GO条目及信号通路的阈值。
1.6 伦理学审查
本实验通过山西中医药大学伦理委员会批准,批号: 2019LL209。
1.7 统计学方法
采用SPSS 25. 0统计软件进行数据分析。计量资料以x±s表示,两组间比较采用独立样本t检验。P<0.05为差异有统计学意义。
2. 结果
2.1 差异表达的miRNA的分析
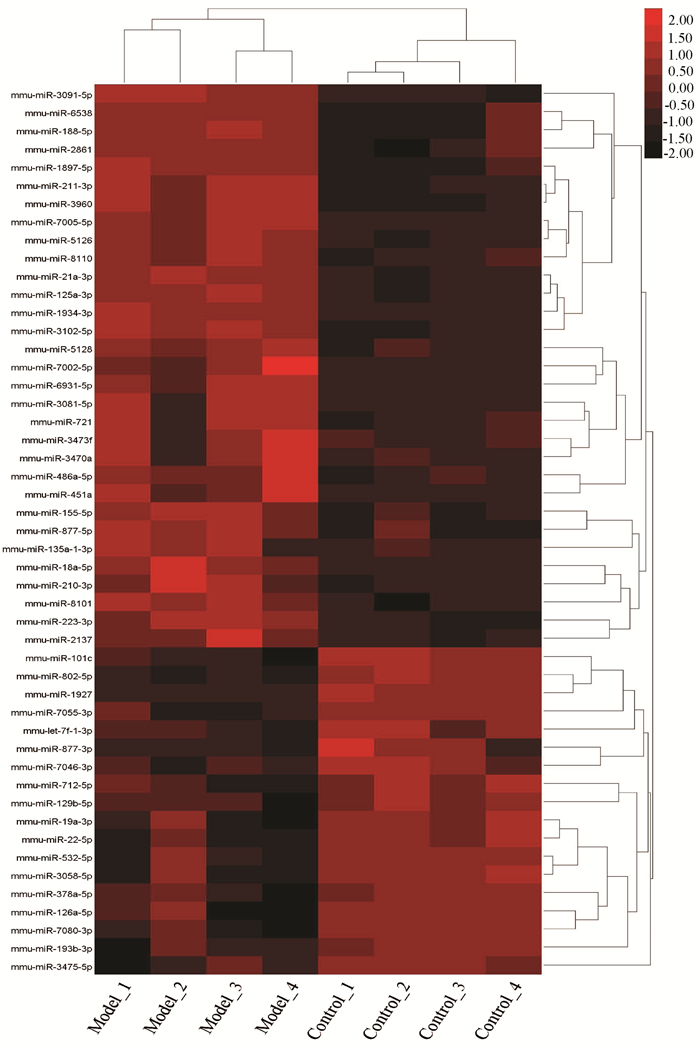
本实验对各实验组差异表达基因分布的不同进行PCA分析,对样本间的关系进行探究。同一分组中的小鼠样本之间的距离相对较近,而不同组中小鼠样本之间的距离相对较远,即模型组中的样本和对照组样本分别各自聚集在一起(图 1),提示组间存在差异,数据满足进一步分析的条件。
以|FC|≥2.0,P≤0.05为的阈值,共筛选出31个表达上调miRNA和18个表达下调miRNA。差异表达miRNA的分布结果见图 2~3: 上调miRNA、下调miRNA与不符合阈值的miRNA明显分开,且各自集中在不同的区域,表明筛选的差异表达miRNA具有可靠性。同时,上调miRNA、下调miRNA与阈值距离越远,代表基因的差异表达越显著,与AIH关系越紧密。图 3结果显示,模型组和对照组各自聚到一个集中的区域,表明两个分组之间存在显著差异,提示差异表达miRNA参与AIH发病。
2.2 差异表达miRNA的qRT-PCR验证
筛选6个差异表达miRNA(上调的是mmu-miR-21a-3p、mmu-miR-1934-3p、mmu-miR-188-5p、下调的是mmu-miR-22-5p、mmu-miR-7055-3p、mmu-miR-126a-5p)进行qRT-PCR扩增,验证微阵列数据的可靠性,PCR引物序列见表 1。结果显示,在表达上调的miRNA中,mmu-miR-21a-3p(20.69倍)的表达水平最高,其次是mmu-miR-188-5p(19.54倍)、mmu-miR-1934-3p(16.40倍);在表达下调的miRNA中,mmu-miR-126a-5p(4.34倍)下调程度最明显,其次是mmu-miR-22-5p(3.49倍)mmu-miR-7055-3p(2.61倍),差异均有统计学意义(P值均<0.01)。与对照组比较,模型组小鼠肝脏内mmu-miR-21a-3p、mmu-miR-1934-3p与mmu-miR-188-5p mRNA表达水平升高,差异有统计学意义(P值均<0. 05);而mmu-miR-22-5p、mmu-miR-7055-3p与mmu-miR-126a-5p mRNA表达水平降低,差异有统计学意义(P值均<0.05)(表 2)。qRT-PCR检测到的含量变化趋势与芯片筛选结果一致,说明微阵列筛选结果是可靠的,满足之后分析条件。
表 1 PCR引物序列miRNA 序列 mmu-miR- 21a-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGACAGCCC-3′ F:5′-ACACTCCAGCTGGGCAACAGCAGTCGAT-3′ mmu-miR- 1934-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCACCAGC-3′ F:5′-ACACTCCAGCTGGGAGGATGACGGTGGGG-3′ mmu-miR- 188-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTCCA-3′ F:5′-ACACTCCAGCTGGGCATCCCTTGCATGG-3′ mmu-miR- 22-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAAAGCTT-3′ F:5′-ACACTCCAGCTGGGAGTTCTTCAGTGGC-3′ mmu-miR- 7055-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTGGTGGG-3′ F:5′-ACACTCCAGCTGGGTTGCTACTTTGATAC-3′ mmu-miR- 126a-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCGTAC-3′ F:5′-ACACTCCAGCTGGGCATTATTACTTTTGG-3′ All R:5′-TGGTGTCGTGGAGTCG-3′ U6 F:5′-CTCGCTTCGGCAGCACA-3′ R:5′-AACGCTTCACGAATTTGCGT-3′ 表 2 各组小鼠肝脏差异表达基因mRNA相对水平的比较组别 mmu-miR-21a-3p mmu-miR-1934-3p mmu-miR-188-5p mmu-miR-22-5p mmu-miR-7055-3p mmu-miR-126a-5p 对照组 1.03±0.31 1.03±0.45 1.18±0.29 -1.27±0.36 -1.2±0.21 -1.1±0.32 模型组 20.68±3.00 16.4±2.31 19.5±2.01 -3.48±0.80 -2.6±0.31 -4.3±1.08 t值 88.870 25.820 45.490 4.739 2.148 11.33 P值 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 2.3 靶基因预测和GO富集分析
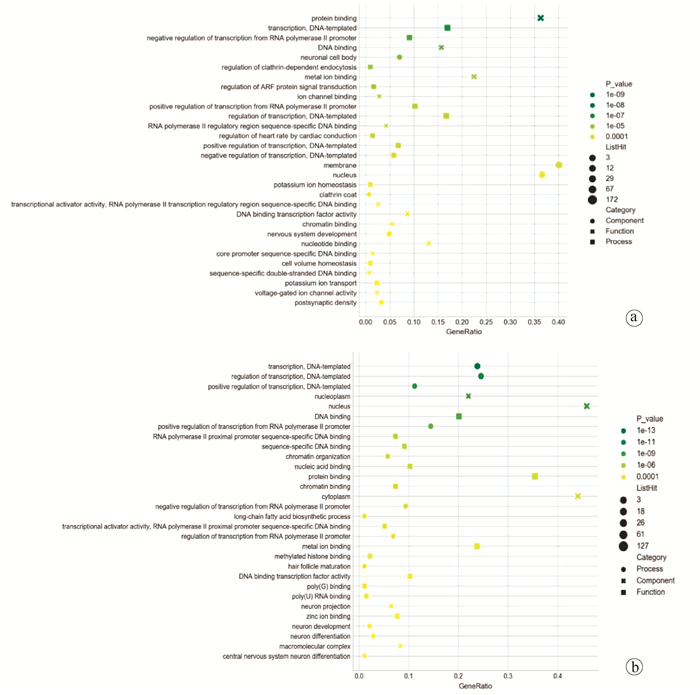
通过miRWalk在线数据库和miRDB在线数据库进行预测,共得上述49个miRNA的交集基因靶点959个。其中上调的miRNA交集基因靶点601个,下调的miRNA交集基因靶点358个。通过GO数据库,设置检索条件(P≤0.05,FDR≤0.05),分析发现31个上调的miRNA的601个靶基因被注释在33个GO项,其中包括10条BP,7项CC,16项MF。根据P值大小按升序排列,选择前30个GO项绘制气泡图与条形图(图 4a)。GO分析结果表明,与对照组比较,模型组中上调的miRNA参与的BP主要包括"transcription,DNA-templated"(P=2.36×10-7)等;这些靶点与CC的"neuronal cell body"(P=5.99×10-6)等关系密切;同时这些靶点主要富集的MF包括" DNA binding"(P=1.47×10-6)等,上述GO富集分析结果差异均有统计学意义(P值<0.05)。18个下调的miRNA的358个靶基因被注释在37个GO项,其中包括14条BP,5项CC,18项MF。根据P值从小到大排列,选择前30个GO项绘制气泡图和条形图(图 4b)。GO分析结果表明,与对照组比较,模型组中下调的miRNA参与的BP主要有"regulation of transcription, DNA-templated"(P=1.64×10-11);这些靶点与CC的"nucleoplasm"(P=4.30×10-10)等关系密切;同时这些靶点与"RNA polymerase Ⅱ proximal promoter sequence-specific DNA binding"(P=2.49×10-6)等功能有关,上述GO富集分析结果差异均有统计学意义(附录1~2)。
2.4 KEGG信号通路分析
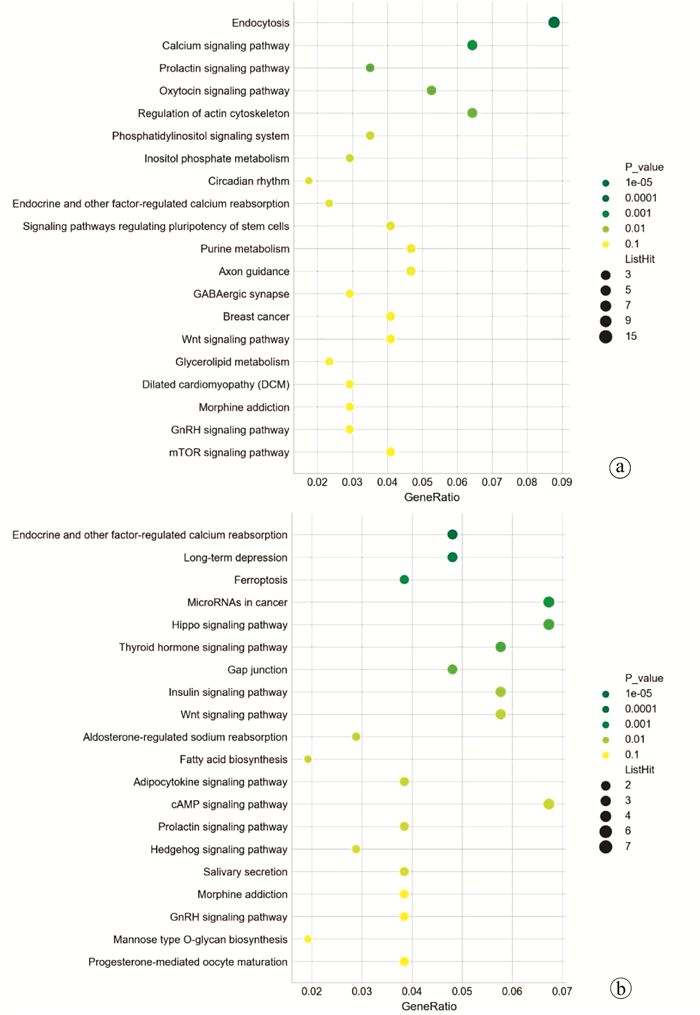
上调的miRNA及下调的miRNA的靶基因可以分别共富集于20条信号通路及26条信号通路。根据前20条路径以P值的升序排列,绘制得出气泡图(图 5)。KEGG通路分析结果表明,与对照组比较,模型组中上调靶点主要涉及"Endocytosis"(P=0.0004)等,下调靶点主要涉及"Hippo信号通路"(P=0.004),同时与"Ferroptosis"(P=0.0018)等疾病基因信号通路有关。其中"Wnt信号通路"(上调P值=0.04,下调P值=0.12),与上调和下调的靶点均密切相关。上述KEGG富集分析结果差异均有统计学意义(附录3~4)。
3. 讨论
AIH的临床特征为转氨酶水平升高、界面性肝炎、高丙种球蛋白血症和特异性自身抗体的存在[7]。主要影响女性(男女比例为1∶4),常见于欧美发达国家,我国的发病率也呈现升高趋势[8]。目前,AIH的确切发病机制仍不清楚,但是遗传易感性、分子模拟机制等因素诱导自身免疫平衡状态的破坏,进而导致肝脏自身抗原特异性细胞攻击肝细胞,造成肝损伤被认为是疾病发生的关键病理因素[9]。本研究发现,AIH的发病过程复杂,多个差异表达miRNA共同参与了AIH的发病。
miRNA是由19~24个核苷酸组成的小的内源性RNA分子,通过碱基配对到互补位点来控制靶向RNA的翻译和转录,被认为的调节基因表达的重要因子之一。miRNA作为一种新的生物标志物,正逐渐成为诊断和预测各种肝病预后的有效的工具[1, 10]。其中miRNA芯片作为一种强大便捷的高通量检测技术,可以同时检测不同分组样品中差异表达miRNA,快速可靠地识别具有统计学和生物学意义的靶标,有利于发掘和研究与疾病相关的信号传导途径。PCA分析法可以从不同维度展现样本间的关系,理想的PCA结果是同组样本距离较近,不同组样本相对离散。本研究PCA结果显示,模型组中的样本和对照组样本分别各自聚集在一起,提示组间存在差异,满足下一阶段的分析条件。接下来笔者团队利用miRNA微阵列芯片,在模型组中检测到31个表达上调的miRNA和18个表达下调的miRNA,并通过火山图和聚类热图对不同样品中差异表达miRNA分布情况进行可视化观察和数据分析,表明筛选的差异表达miRNA具有可靠性。同时,笔者团队选择6个差异表达miRNA进行qRT-PCR验证,验证结果与微阵列分析获得的数据趋势相同,证实了微阵列筛选数据的可信度。目前研究表明,这些差异表达的miRNA在肝脏炎症或自身免疫性疾病中具有重要调节潜力。31个上调的miRNA中,如miR-223-3p在临床研究中已显示出减轻炎症反应的效果,其高度包裹在源自骨间充质干细胞(mesenchymal stem cells,MSC)的外泌体中,MSC外泌体能够充当载体,将miRNA传递到细胞中。研究[11]发现,MSC外泌体可以成功地与miR-223-3p结合,并将miR-223-3p传递到巨噬细胞中,对炎症相关基因STAT3的表达起到负性调节作用,因此miR-223-3p可以抑制肝脏和巨噬细胞中的炎症细胞因子释放,减轻炎症反应。研究[12]发现,与野生型小鼠相比,ConA干预后的miR-155-/-小鼠肝转氨酶和促炎介质的水平更高,此外miR-155缺乏症还通过含有SH2结构域的肌醇5-磷酸酶1导致调节性T淋巴细胞募集受损,通过骨髓移植恢复基因敲除小鼠的miR-155,可以显着减少肝坏死和炎症,提示miR-155在AIH发生发展中具有保护作用。在表达下调的miRNA中,如miR-let-7a通过靶向IL-6R抑制LPS诱导的软骨细胞炎症损伤,减少了细胞凋亡并降低炎症反应[13]。
miRNA主要通过与靶mRNA的结合,或促使mRNA降解,或阻碍其翻译,从而抑制目的基因的表达。本研究中,笔者团队通过2个数据库miRWalk和miRDB的交集筛选了差异表达miRNA的潜在靶标,共获得959个靶基因(上调601个,下调个358),每个差异表达的miRNA都调节了一系列相关的靶基因。GO富集分析结果提示部分上调的miRNA会影响RNA在DNA模板上的细胞合成并且影响RNA聚合酶Ⅱ启动子转录频率、速率或程度的降低。这些靶点与CC的"neuronal cell body"等关系密切,并且可以与DNA或者任何蛋白质(包括蛋白质复合物)选择性和非共价相互作用。下调的miRNA参与的BP主要有"regulation of transcription,DNA-templated",反映出下调miRNA与调节细胞DNA模板转录的频率、速率或程度等过程密切相关。这些靶点与"nucleoplasm"等关系密切,并且具备"RNA polymeraseⅡproximal promoter sequence-specific DNA binding"等功能。
KEGG通路分析结果表明"Wnt信号通路"富集了上调和下调多数关键基因,可能与AIH有重要联系。典型的Wnt信号通路高度保守,是肝损伤的重要调节器[14]。表没食子儿茶素没食子酸酯(epigallocatechingallate,EGCG)是从植物绿茶中提取的儿茶素类单体,具有抑制炎性反应的作用。研究[15]发现,使用EGCG干预AIH小鼠,可以通过下调肝脏中Wnt1、β-catenin的表达,明显改善AIH小鼠肝细胞的损伤。此外,有学者[16]提出,Wnt/β-catenin可能通过与Hedgehog、HIF通路之间的串扰作用维持肝内稳态,有助于建立疾病模型、提高筛选药物的能力。笔者团队研究发现,上调靶点主要涉及"mTOR信号通路"和内吞作用等。先前研究[17-18]表明,mTOR的激活会通过影响自噬促进肝损伤,一些miRNA通过在转录后水平上干扰基因表达而参与自噬的调控。例如,miR-155通过下调mTOR信号诱导自噬体的形成,而且破坏自噬体与溶酶体的融合。miR-204会减弱肝细胞癌的肿瘤自噬作用。miR-101是一种自噬抑制剂,在肝细胞癌中抑制了癌细胞凋亡。同时,miR-101可显着改善CCl4所致小鼠肝功能损伤,并通过下调PI3K/Akt/mTOR信号转导通路参与抗纤维化机制[19]。肝细胞内吞作用是一个高度动态的过程,负责将各种不同的受体配体复合物、营养因子、脂质以及许多不同的病原体内化,内吞途径与脂质筏、小窝等相关。肝窦内皮细胞(sinusoidal endothelial cell,SEC)是最具内吞活性的细胞之一,可有效吸收降解外源和代谢产物[20]。ConA诱导的AIH模型中,ConA注射后首先与SEC表面的甘露糖腺结合,导致SEC膜破裂和细胞质消失,SEC的迅速分离促进了ConA与Kupffer细胞的结合(4 h左右),T淋巴细胞通过交联其T淋巴细胞抗原受体而被激活,CD4+T淋巴细胞的活化明显增强,促进了促炎因子大量分泌,从而导致肝损伤的发生[21]。本实验KEGG通路分析发现下调靶点主要涉及"Hippo信号通路",同时与"Ferroptosis"等疾病基因密切相关。研究[22]表明,"Hippo信号通路"在维持肝组织稳态中起着关键作用,其失调会损害肝再生,并导致肝脏过度生长和肿瘤发生。目前已证实Hippo信号通路与某些炎症因子的信号通路有密切关系。转化生长因子β是一种重要的炎症因子,可以与Hippo信号通路相互作用,在核内聚集形成转录调节器,通过调节相关靶基因的转录,参与炎症反应。香芹酚可以通过靶向CCl4诱导大鼠肝纤维化的Hippo和转化生长因子β信号通路改善肝纤维化的进展[23]。Ferroptosis是一种公认的调节性细胞死亡过程,与氧化还原状态、新陈代谢和人类健康有关,被认为是对广泛的脂质过氧化的防御机制,可能破坏细胞膜的完整性,最终导致毒性细胞损伤。有证据[24]表明,Ferroptosis在调节多种肝脏疾病的发生和发展中起着关键作用。肝星状细胞(HSC)中储存有大量的铁离子,为HSC可能发生Ferroptosis提供了条件。异甘草酸镁通过促进铁和脂质过氧化物的积累会显著诱导HSC发生Ferroptosis,减轻CCl4诱导的肝纤维化大鼠的肝损伤程度。而特异性抑制剂ferrostatin-1通过抑制Ferroptosis可以消除异甘草酸镁诱导的抗纤维化作用。同样,青蒿琥酯可以通过调节HSC中的铁蛋白介导的Ferroptosis减轻肝纤维化[25]。此外,临床研究[26]发现自身免疫性肝病患者血清中的铁调素浓度比正常人低,长期肝内铁大量堆积还会导致铁过载,提示Ferroptosis参与了肝脏疾病的发生。差异表达miRNA的许多靶点基因富集在不同通路,为AIH研究提供了更多的可能。
综上所述,本研究使用微阵列芯片筛选了ConA诱导的AIH小鼠模型中表达上调和下调的miRNA,并通过生物信息学方法对这些miRNA的靶基因进行功能分析,分别探讨了其靶基因的生物学功能,证实了miRNA作为一类特殊生物标志物在AIH发病机理中的重要作用。随着对miRNA与AIH发病机制相关性的深入研究,将会为AIH的临床治疗提供新的思路和方案。
-
表 1 PCR引物序列
miRNA 序列 mmu-miR- 21a-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGACAGCCC-3′ F:5′-ACACTCCAGCTGGGCAACAGCAGTCGAT-3′ mmu-miR- 1934-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCACCAGC-3′ F:5′-ACACTCCAGCTGGGAGGATGACGGTGGGG-3′ mmu-miR- 188-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTCCA-3′ F:5′-ACACTCCAGCTGGGCATCCCTTGCATGG-3′ mmu-miR- 22-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAAAGCTT-3′ F:5′-ACACTCCAGCTGGGAGTTCTTCAGTGGC-3′ mmu-miR- 7055-3p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTGGTGGG-3′ F:5′-ACACTCCAGCTGGGTTGCTACTTTGATAC-3′ mmu-miR- 126a-5p R:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCGTAC-3′ F:5′-ACACTCCAGCTGGGCATTATTACTTTTGG-3′ All R:5′-TGGTGTCGTGGAGTCG-3′ U6 F:5′-CTCGCTTCGGCAGCACA-3′ R:5′-AACGCTTCACGAATTTGCGT-3′ 表 2 各组小鼠肝脏差异表达基因mRNA相对水平的比较
组别 mmu-miR-21a-3p mmu-miR-1934-3p mmu-miR-188-5p mmu-miR-22-5p mmu-miR-7055-3p mmu-miR-126a-5p 对照组 1.03±0.31 1.03±0.45 1.18±0.29 -1.27±0.36 -1.2±0.21 -1.1±0.32 模型组 20.68±3.00 16.4±2.31 19.5±2.01 -3.48±0.80 -2.6±0.31 -4.3±1.08 t值 88.870 25.820 45.490 4.739 2.148 11.33 P值 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 -
[1] KRAWITT EL. Autoimmune hepatitis[J]. N Engl J Med, 2006, 354(1): 54-66. DOI: 10.1056/NEJMra050408. [2] PAPE S, SCHRAMM C, GEVERS TJ. Clinical management of autoimmune hepatitis[J]. United European Gastroenterol J, 2019, 7(9): 1156-1163. DOI: 10.1177/2050640619872408. [3] FRANCQUE S, VONGHIA L, RAMON A, et al. Epidemiology and treatment of autoimmune hepatitis[J]. Hepat Med, 2012, 4: 1-10. DOI: 10.2147/HMER.S16321. [4] LOOSEN SH, SCHUELLER F, TRAUTWEIN C, et al. Role of circulating microRNAs in liver diseases[J]. World J Hepatol, 2017, 9(12): 586-594. DOI: 10.4254/wjh.v9.i12.586. [5] HAO JH, CHEN H, GAO Y, et al. Effects of Saikosaponin d on differentially expressed genes CTLA-4, IL-10 and IL-17 in mice with autoimmune hepatitis[J]. J Shanghai Jiaotong Univ (Med Sci), 2020, 40(3): 303-309. DOI: 10.3969/j.issn.1674-8115.2020.03.005.郝健亨, 陈浩, 高艳, 等. 柴胡皂苷d对自身免疫性肝炎小鼠差异表达基因CTLA-4、IL-10和IL-17的影响[J]. 上海交通大学学报(医学版), 2020, 40(3): 303-309. DOI: 10.3969/j.issn.1674-8115.2020.03.005. [6] CHEN H, HAO JH, LI ZC, et al. Screening for differentially expressed genes in mice with autoimmune hepatitis and effect of saikosaponin-D on the expression of several differentially expressed genes[J]. J Clin Hepatol, 2020, 36(4): 840-846. DOI: 10.3969/j.issn.1001-5256.2020.04.026.陈浩, 郝健亨, 李振城, 等. 自身免疫性肝炎小鼠模型差异表达基因的筛查及柴胡皂苷D对部分差异表达基因表达的影响[J]. 临床肝胆病杂志, 2020, 36(4): 840-846. DOI: 10.3969/j.issn.1001-5256.2020.04.026. [7] LIU Y, CHEN H, HAO JH, et al. Characterization and functional prediction of the microRNAs differentially expressed in a mouse model of concanavalin a-induced autoimmune hepatitis[J]. Int J Med Sci, 2020, 17(15): 2312-2327. DOI: 10.7150/ijms.47766. [8] SUCHER E, SUCHER R, GRADISTANAC T, et al. Autoimmune hepatitis-immunologically triggered liver pathogenesis-diagnostic and therapeutic strategies[J]. J Immunol Res, 2019, 2019: 9437043. DOI: 10.1155/2019/9437043. [9] THAN NN, JEFFERY HC, OO YH. Autoimmune hepatitis: Progress from global immunosuppression to personalised regulatory T cell therapy[J]. Can J Gastroenterol Hepatol, 2016, 2016: 7181685. Doi: 10.1155/2016/7181685. [10] HAYES CN, CHAYAMA K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma[J]. Int J Mol Sci, 2016, 17(3): 280. DOI: 10.3390/ijms17030280. [11] LU FB, CHEN DZ, CHEN L, et al. Attenuation of experimental autoimmune hepatitis in mice with bone mesenchymal stem cell-derived exosomes carrying microRNA-223-3p[J]. Mol Cells, 2019, 42(12): 906-918. DOI: 10.14348/molcells.2019.2283. [12] BLAYA D, AGUILAR-BRAVO B, HAO F, et al. Expression of microRNA-155 in inflammatory cells modulates liver injury[J]. Hepatology, 2018, 68(2): 691-706. DOI: 10.1002/hep.29833. [13] SUI C, ZHANG L, HU Y. MicroRNA-let-7a inhibition inhibits LPS?induced inflammatory injury of chondrocytes by targeting IL6R[J]. Mol Med Rep, 20(3): 2633-2640. DOI: 10.3892/mmr.2019.10493. [14] RUSSELL JO, MONGA SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology[J]. Annu Rev Pathol, 2018, 13: 351-378. DOI: 10.1146/annurev-pathol-020117-044010. [15] ZHOU D, YU Y, AN Y, et al. EGCG mediated Wnt/β-catenin signaling pathway improves liver damage in mice with immune hepatitis[J]. Anatomy Res 2020, 42 (1): 38-43. DOI: GDJP.0.2020-01-007周丹, 于洋, 安洋, 等. 表没食子儿茶素没食子酸酯通过Wnt/β-catenin信号通路改善免疫性肝炎小鼠肝损伤[J]. 解剖学研究, 2020, 42(1): 38-43. DOI: GDJP.0.2020-01-007. [16] ZHAO L, JIN Y, DONAHUE K, et al. Tissue repair in the mouse liver following acute carbon tetrachloride depends on injury-induced Wnt/β-catenin signaling[J]. Hepatology, 2019, 69(6): 2623-2635. DOI: 10.1002/hep.30563. [17] SONG H, DU C, WANG X, et al. MicroRNA-101 inhibits autophagy to alleviate liver ischemia/reperfusion injury via regulating the mTOR signaling pathway[J]. Int J Mol Med, 2019, 43(3): 1331-1342. DOI: 10.3892/ijmm.2019.4077. [18] LEI Y, WANG QL, SHEN L, et al. MicroRNA-101 suppresses liver fibrosis by downregulating PI3K/Akt/mTOR signaling pathway[J]. Clin Res Hepatol Gastroenterol, 2019, 43(5): 575-584. DOI: 10.1016/j.clinre.2019.02.003. [19] SZEKERCZÉS T, GÓGL A, ILLYÉS I, et al. Autophagy, mitophagy and microRNA expression in chronic hepatitis C and autoimmune hepatitis[J]. Pathol Oncol Res, 2020, 26(4): 2143-2151. DOI: 10.1007/s12253-020-00799-y. [20] SCHROEDER B, MCNIVEN MA. Importance of endocytic pathways in liver function and disease[J]. Compr Physiol, 2014, 4(4): 1403-1417. DOI: 10.1002/cphy.c140001. [21] WANG HX, LIU M, WENG SY, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis[J]. World J Gastroenterol, 2012, 18(2): 119-125. DOI: 10.3748/wjg.v18.i2.119. [22] HONG L, CAI Y, JIANG M, et al. The Hippo signaling pathway in liver regeneration and tumorigenesis[J]. Acta Biochim Biophys Sin (Shanghai), 2015, 47(1): 46-52. DOI: 10.1093/abbs/gmu106. [23] MOHSENI R, KARIMI J, TAVILANI H, et al. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl4)-induced liver fibrosis in rats[J]. Immunopharmacol Immunotoxicol, 2019, 41(1): 163-171. DOI: 10.1080/08923973.2019.1566926. [24] SUI M, JIANG X, CHEN J, et al. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway[J]. Biomed Pharmacother, 2018, 106: 125-133. DOI: 10.1016/j.biopha.2018.06.060. [25] KONG Z, LIU R, CHENG Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway[J]. Biomed Pharmacother, 2019, 109: 2043-2053. DOI: 10.1016/j.biopha.2018.11.030. [26] LYBEROPOULOU A, CHACHAMI G, GATSELIS NK, et al. Low serum hepcidin in patients with autoimmune liver diseases[J]. PLoS One, 2015, 10(8): e0135486. DOI: 10.1371/journal.pone.0135486. 期刊类型引用(4)
1. 胡毅龙,赵怡楠,张双丽,邱广楠,冯艺凡,苗明三,苗晋鑫. 基于数据挖掘的急性肝损伤动物模型应用分析. 中国比较医学杂志. 2024(02): 89-100 .  百度学术
百度学术2. 郑通通,杨妮,徐建良,盛国光,叶雯,熊明珠. 基于高通量测序探讨海珠益肝加味方对自身免疫性肝炎小鼠miRNA表达谱的影响. 中医学报. 2024(12): 2493-2499 .  百度学术
百度学术3. 李聪,周威. 芍药苷对伴刀豆球蛋白诱导的小鼠急性肝损伤的保护作用. 中国临床药理学杂志. 2022(05): 427-431 .  百度学术
百度学术4. 曾鑫,刘妙华,熊祎,闫宫花,刘端勇,殷玉婷,周步高. 姜黄素对AIH小鼠肠道菌群失衡的改善作用. 中华中医药学刊. 2022(10): 68-71+266-268 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 5090 KB)
PDF下载 ( 5090 KB)


 下载:
下载:





 下载:
下载:

 百度学术
百度学术






