肝动脉灌注化疗及其综合治疗方案对中晚期肝细胞癌患者的临床疗效及预后因素分析
DOI: 10.3969/j.issn.1001-5256.2023.07.013
Efficacy of hepatic arterial infusion chemotherapy and its multimodality therapeutic regimens in treatment of patients with advanced hepatocellular carcinoma and related prognostic factors
-
摘要:
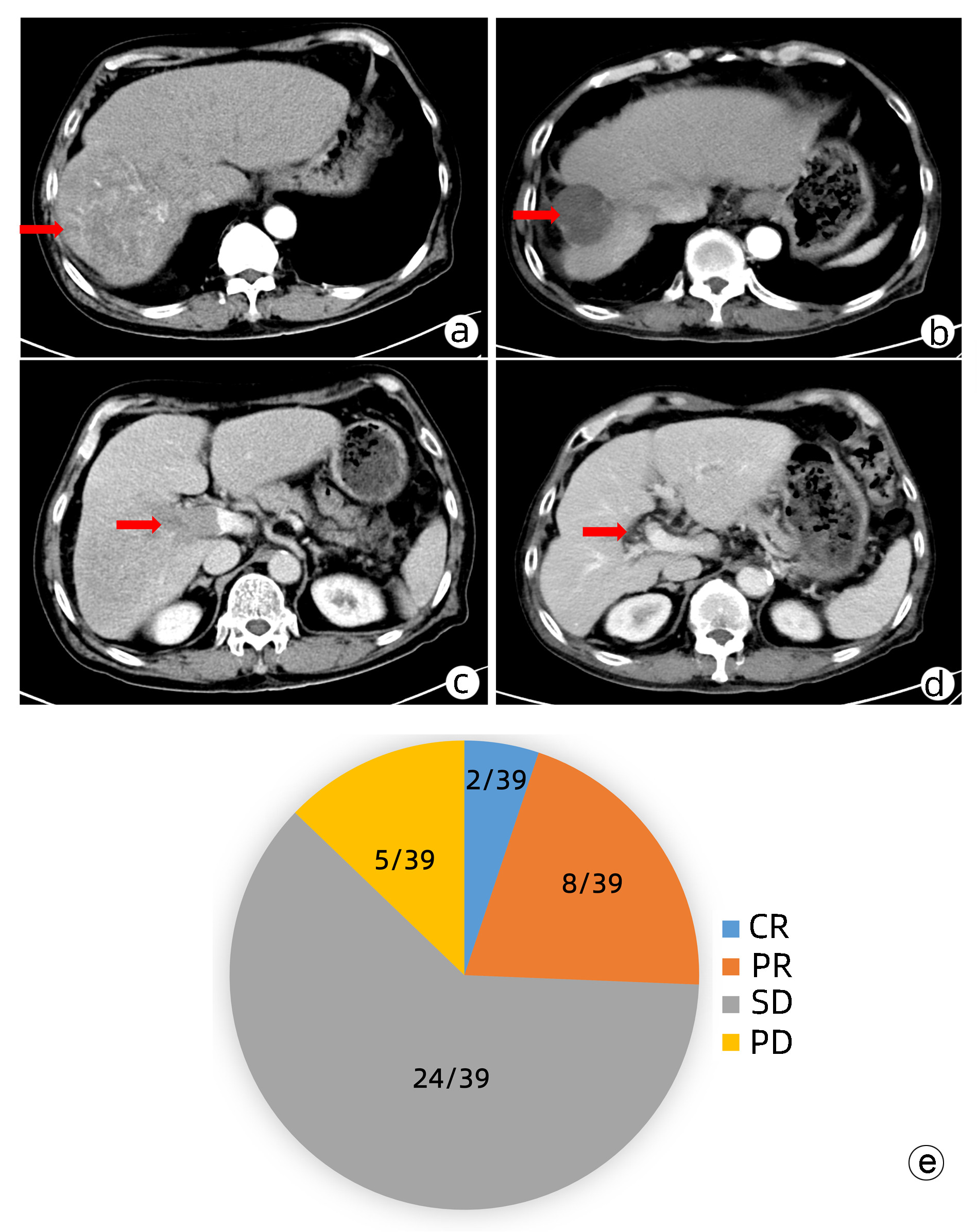
目的 本研究旨在观察FOLFOX方案持续肝动脉灌注化疗(HAIC)及其综合治疗方案对中晚期肝细胞癌患者的临床疗效并分析影响预后的因素。 方法 回顾性收集南方医科大学南方医院2018年9月—2021年11月行FOLFOX方案持续HAIC的66例中晚期肝细胞癌患者临床资料。观察治疗后患者的客观缓解率、疾病控制率、中位无疾病进展生存时间(mPFS)和中位生存时间(mOS)并记录治疗相关不良反应。针对伴有门静脉癌栓的患者,评价治疗对门静脉癌栓的疗效。采用Kaplan-Meier法进行生存分析。采用Cox回归分析影响预后的因素。 结果 按照RECIST1.1标准,FOLFOX-HAIC及其综合治疗方案治疗66例中晚期肝细胞癌患者的客观缓解率和疾病控制率分别为33.3%(22/66)、86.4%(57/66),mPFS和mOS分别为8.2个月和22.1个月。其中39例合并门静脉癌栓的肝癌患者中完全缓解2例,部分缓解8例,稳定24例,进展5例。客观缓解率为25.6%(10/39),疾病控制率为87.2%(34/39)。不良反应主要为消化道反应16.7%(11/66)、发热12.1%(8/66)、肝区疼痛10.6%(7/66)、骨髓抑制3.0%(2/66)和造影剂过敏3.0%(2/66)。无Ⅳ级以上的毒副反应。无并发症导致的死亡。Cox分析显示肝外转移(HR=2.668, 95% CI:1.357~5.245)和凝血酶原时间(HR=1.282, 95%CI:1.080~1.630)是影响患者PFS的独立危险因素(P值均<0.05), AST水平(HR=1.008, 95%CI:1.002~1.013)和凝血酶原时间(HR=1.303, 95%CI:1.046~1.630)是影响患者OS的独立危险因素(P值均<0.05)。 结论 FOLFOX-HAIC及其综合治疗方案治疗中晚期肝细胞癌有一定的疗效,不良反应可控。 Abstract:Objective To investigate the efficacy of continuous hepatic arterial infusion chemotherapy (HAIC) with the FOLFOX regimen and its multimodality therapeutic regimen in the treatment of patients with advanced hepatocellular carcinoma, as well as the influencing factors for prognosis. Methods A retrospective analysis was performed for the clinical data of 66 patients with advanced hepatocellular carcinoma who received continuous HAIC with FOLFOX regimen in Nanfang Hospital, Southern Medical University, from September 2018 to November 2021. The patients were observed in terms of objective response rate (ORR), disease control rate (DCR), median progression-free survival (mPFS), and median overall survival (mOS) after treatment, and treatment-related adverse reactions were recorded. For the patients with portal vein tumor thrombus, the effect of the treatment on portal vein tumor thrombus was assessed. The Kaplan-Meier method was used for survival analysis, and the Cox regression analysis was used to investigate the influencing factors for prognosis. Results According to the RECIST1.1 criteria, FOLFOX-HAIC and its multimodality therapeutic regimen achieved an ORR of 33.3% (22/66) and a DCR of 86.4% (57/66) in the treatment of 66 patients with advanced hepatocellular carcinoma, with an mPFS time of 8.2 months and an mOS time of 22.1 months. Among the 39 patients with portal vein tumor thrombus, 2 achieved complete remission, 8 achieved partial remission, 24 achieved stable disease, and 5 had disease progression, with an ORR of 25.6% (10/39) and a DCR of 87.2% (34/39). The main adverse reactions included gastrointestinal reactions (16.7%, 11/66), pyrexia (12.1%, 8/66), liver area pain (10.6%, 7/66), bone marrow suppression (3.0%, 2/66), and contrast agent allergy (3.0%, 2/66), and there were no grade > Ⅳ toxic or side effects or deaths caused by such complications. The Cox regression analysis showed that extrahepatic metastasis (hazard ratio [HR]=2.668, 95% confidence interval [CI]: 1.357-5.245, P < 0.05) and prothrombin time (PT) (HR=1.282, 95%CI: 1.080-1.630, P < 0.05) were independent risk factors for PFS, and aspartate aminotransferase level (HR=1.008, 95%CI: 1.002-1.013, P < 0.05) and PT (HR=1.303, 95%CI: 1.046-1.630, P < 0.05) were independent risk factors for OS. Conclusion FOLFOX-HAIC and its multimodality therapeutic regimen has a certain clinical effect with controllable adverse reactions in the treatment of advanced hepatocellular carcinoma. -
Key words:
- Carcinoma, Hepatocellular /
- Molecular Targeted Therapy /
- Prognosis
-
表 1 患者基线资料
Table 1. Baseline characteristics of HCC patients
指标 HAIC
(n=11)HAIC+TKI
(n=14)HAIC+TKI+PD-1/PD-L1
(n=41)合计 年龄(岁) 47.55±12.73 50.30±10.29 46.71±9.87 47.61±10.40 性别[例(%)] 男 10(90.9) 13(92.9) 39(95.1) 62(93.9) 女 1(9.1) 1(7.1) 2(4.9) 4(6.1) Child-Pugh分级[例(%)] A级 11(100.00) 9(64.3) 28(68.3) 48(72.7) B级 0(0.0) 5(35.7) 13(31.7) 18(27.3) BCLC分期[例(%)] B 4(36.4) 5(35.7) 9(22.0) 18(27.3) C 7(63.6) 9(64.3) 32(78.0) 48(72.7) AFP[例(%)] <400 ng/mL 8(72.7) 4(28.6) 12(29.3) 24(36.4) ≥400 ng/mL 3(27.3) 10(71.4) 29(70.7) 42(63.6) ALT(U/L) 47.00(24.00~96.00) 44.00(26.50~76.00) 36.00(26.00~55.50) 38.50(25.00~60.25) AST(U/L) 50.00(26.00~151.00) 54.00(43.00~98.25) 45.00(36.00~67.50) 48.50(35.00~74.25) Alb(g/L) 38.17±4.64 36.14±4.60 36.02±5.21 36.40±4.99 TBil(μmol/L) 13.20(8.10~21.00) 15.85(8.73~27.00) 15.40(12.10~23.45) 15.40(10.58~21.83) PLT(×109/L) 218.73±149.46 183.93±91.88 217.20±110.75 210.39±113.42 PT(s) 11.61±1.27 11.87±1.16 12.37±1.71 12.14±1.55 肿瘤特点[例(%)] 肝外转移 5(45.5) 6(42.9) 16(39.0) 27(40.9) 门静脉侵犯 5(45.5) 8(57.1) 26(63.4) 39(59.1) 单个肿瘤 0(0.0) 1(7.1) 4(9.8) 5(7.6) 多个肿瘤 11(100.0) 13(92.9) 37(90.2) 61(92.4) 肿瘤最大径<7 cm 5(45.5) 6(42.9) 11(26.8) 22(33.3) 肿瘤最大径≥7 cm 6(54.5) 8(57.1) 30(73.2) 44(66.7) 合并高血压或糖尿病 4(36.4) 2(14.3) 10(24.4) 16(24.2) HBV DNA[例(%)] 阳性 4(36.4) 6(42.9) 26(63.4) 36(54.5) 阴性 7(63.6) 8(57.1) 15(36.6) 30(45.5) 表 2 患者的最佳肿瘤疗效评价结果
Table 2. Best tumor response in patients
组别 例数 CR (例) PR (例) SD (例) PD (例) ORR (%) DCR (%) HAIC组 11 0 2 8 1 18.1 90.9 HAIC+TKI组 14 0 3 9 2 21.4 85.7 HAIC+TKI+PD-1/PD-L1组 41 0 17 18 6 41.5 85.4 合计 66 0 22 35 9 33.3 86.4 表 3 影响患者PFS的Cox单因素和多因素分析
Table 3. Univariate and multivariate Cox analysis of risk factors for progression-free survival
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 肝外转移(有vs无) 2.907 1.536~5.500 0.001 2.668 1.357~5.245 0.004 HBV DNA (阳性vs阴性) 2.151 1.126~4.110 0.020 PT 1.254 1.024~1.535 0.028 1.282 1.080~1.630 0.042 表 4 影响患者OS的Cox单因素和多因素分析
Table 4. Univariate and multivariate Cox analysis of risk factors for overall survival
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 年龄 0.964 0.931~0.999 0.042 AST 1.007 1.002~1.013 0.007 1.008 1.002~1.013 0.006 PT 1.276 1.036~1.572 0.022 1.303 1.046~1.630 0.018 -
[1] Global Burden of Disease Liver Cancer Collaboration, AKINYEMIJU T, ABERA S, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015[J]. JAMA Oncol, 2017, 3(12): 1683-1691. DOI: 10.1001/jamaoncol.2017.3055. [2] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [3] ZHENG R, ZENG H, ZHANG S, et al. Estimates of cancer incidence and mortality in China, 2013[J]. Chin J Cancer, 2017, 36(1): 66. DOI: 10.1186/s40880-017-0234-3. [4] WU T, CHEN L. New advances in the precision diagnosis and treatment of liver cancer[J]. J Clin Hepatol, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001.吴彤, 陈磊. 肝癌精准诊疗新进展[J]. 临床肝胆病杂志, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001. [5] LI Z, ZHU JY. Interpretation of Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(5): 1027-1029. DOI: 10.3969/j.issn.1001-5256.2022.05.010.李照, 朱继业. 《原发性肝癌诊疗指南(2022年版)》解读[J]. 临床肝胆病杂志, 2022, 38(5): 1027-1029. DOI: 10.3969/j.issn.1001-5256.2022.05.010. [6] CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase Ⅲ randomised, double-blind, placebo- controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. [7] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. [8] LYU N, KONG Y, PAN T, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin in hepatocellular cancer with extrahepatic spread[J]. J Vasc Interv Radiol, 2019, 30(3): 349-357. e2. DOI: 10.1016/j.jvir.2018.09.004. [9] HE MK, LIANG RB, ZHAO Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma[J]. Ther Adv Med Oncol, 2021, 13: 17588359211002720. DOI: 10.1177/17588359211002720. [10] KOKUDO N, HASEGAWA K, AKAHANE M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines)[J]. Hepatol Res, 2015, 45(2). DOI: 10.1111/hepr.12464. [11] IKEDA M, OKUSAKA T, FURUSE J, et al. A multi-institutional phase Ⅱ trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis[J]. Cancer Chemother Pharmacol, 2013, 72(2): 463-470. DOI: 10.1007/s00280-013-2222-x. [12] NOUSO K, MIYAHARA K, UCHIDA D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan[J]. Br J Cancer, 2013, 109(7): 1904-1907. DOI: 10.1038/bjc.2013.542. [13] Tumor Interventional Expert Committee of Chinese Anti-Cancer Association. Chinese tumor intervention expert consensus on the application principles of transcatheter arterial infusion chemotherapy[J]. J Intervent Radiol, 2017, 26(11): 963-970. DOI: 10.3969/j.issn.1008-794X.2017.11.001.中国抗癌协会肿瘤介入专家委员会. 经导管动脉灌注化疗药物应用原则——中国肿瘤介入专家共识[J]. 介入放射学杂志, 2017, 26(11): 963-970. DOI: 10.3969/j.issn.1008-794X.2017.11.001. [14] ZHAO M. Hepatic arterial infusion Chemotherapy in the Era of Precise[J]. J Sun Yat-Sen Univ(Medical Sciences), 2019, 40(5): 648-656. DOI: 1672-3554(2019)05-0648-09.赵明. 精准医疗时代背景下的肝动脉灌注化疗[J]. 中山大学学报(医学科学版), 2019, 40(5): 648-656. DOI: 1672-3554(2019)05-0648-09. [15] LI QJ, HE MK, CHEN HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A Randomized Phase Ⅲ Trial[J]. J Clin Oncol, 2022, 40(2): 150-160. DOI: 10.1200/JCO.21.00608. [16] SIDAWAY P. FOLFOX-HAIC active in large HCC[J]. Nat Rev Clin Oncol, 2022, 19(1): 5. DOI: 10.1038/s41571-021-00577-y. [17] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [18] Chinese College of Transplant Doctors, Liver Transplantation Group, Chinese Society of Organ Transplantation, Chinese Medical Association. Chinese clinical practice guidelines on liver transplantation for hepatocellular carcinoma (2021edition)[J]. Chin J Dig Surg, 2022, 21(4): 433-443. DOI: 10.3760/cma.j.cn115610-20220316-00135.中国医师协会器官移植医师分会, 中华医学会器官移植学分会肝移植学组. 中国肝癌肝移植临床实践指南(2021版)[J]. 中华消化外科杂志, 2022, 21(4): 433-443. DOI: 10.3760/cma.j.cn115610-20220316-00135. [19] LIU YY. New progress of hepatocellular carcinoma treatment[J]. Chin J Dig Surg, 2022, 21(1): 15-18. DOI: 10.3760/cma.j.cn115610-20220107-00020.刘允怡. 肝细胞癌治疗的新发展[J]. 中华消化外科杂志, 2022, 21(1): 15-18. DOI: 10.3760/cma.j.cn115610-20220107-00020. [20] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [21] Liver Cancer Professional Committee of Chinese Anti-Cancer Association. Chinese expert consensus on hepatic arterial infusion chemotherapy for hepatocellular carcinoma (2021 edition)[J]. J Chin J Dig Surg, 2021, 20(7): 754-759. DOI: 10.3760/cma.j.cn115610-20210618-00288.中国抗癌协会肝癌专业委员会. 肝动脉灌注化疗治疗肝细胞癌中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20(7): 754-759. DOI: 10.3760/cma.j.cn115610-20210618-00288. [22] HE MK, LE Y, LI QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study[J]. Chin J Cancer, 2017, 36(1): 83. DOI: 10.1186/s40880-017-0251-2. [23] HE M, LI Q, ZOU R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial[J]. JAMA Oncol, 2019, 5(7): 953-960. DOI: 10.1001/jamaoncol.2019.0250. [24] MEI J, LI SH, LI QJ, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma[J]. J Hepatocell Carcinoma, 2021, 8: 167-176. DOI: 10.2147/JHC.S298538. [25] MEI J, TANG YH, WEI W, et al. Hepatic arterial infusion chemotherapy combined With PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 618206. DOI: 10.3389/fonc.2021.618206. [26] WU YB, WU ZQ, HUANG JL, et al. Hepatic arterial infusion chemotherapy for primary liver cancer effect of portal vein tumor thrombus[J]. China Health Standard Management, 2022, 13(12): 109-112. DOI: 10.3969/j.issn.1674-9316.2022.12.027.吴义波, 吴卓琼, 黄洁丽, 等. 肝动脉灌注化疗对原发性肝癌合并门静脉癌栓的效果[J]. 中国卫生标准管理, 2022, 13(12): 109-112. DOI: 10.3969/j.issn.1674-9316.2022.12.027. [27] KE YP, YE SG, LU SB. Short-term efficacy of transcatheter arterial embolization combined with FOLFOX4 regimen of continuous arterial infusion chemotherapy on hepatocellular carcinoma patients with portal vein tumor thrombus[J]. China Med Pharm, 2022, 12(16): 156-159. DOI: 10.3969/j.issn.2095-0616.2022.16.040.柯映平, 叶绍光, 卢舜彬. 肝动脉栓塞术联合FOLFOX4方案持续动脉灌注化疗对肝细胞癌门静脉癌栓患者的近期疗效[J]. 中国医药科学, 2022, 12(16): 156-159. DOI: 10.3969/j.issn.2095-0616.2022.16.040. -



 PDF下载 ( 2254 KB)
PDF下载 ( 2254 KB)


 下载:
下载: