TXNRD1在肝细胞癌中活性氧相关细胞凋亡导致多药耐药性中的作用
DOI: 10.3969/j.issn.1001-5256.2022.02.022
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:章爱斌负责文章整体构思和设计;李大志负责数据分析,撰写论文;黄俊杰参与撰写及修改论文;郑树森、章爱斌负责指导撰写文章并最终定稿。
Role of thioredoxin reductase 1 in multidrug resistance caused by reactive oxygen species-related cell apoptosis in hepatocellular carcinoma
-
摘要:
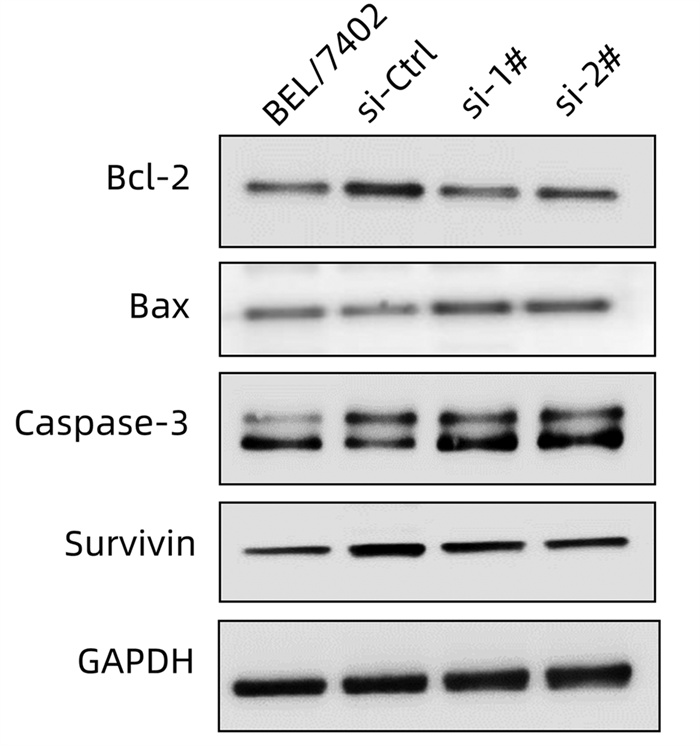
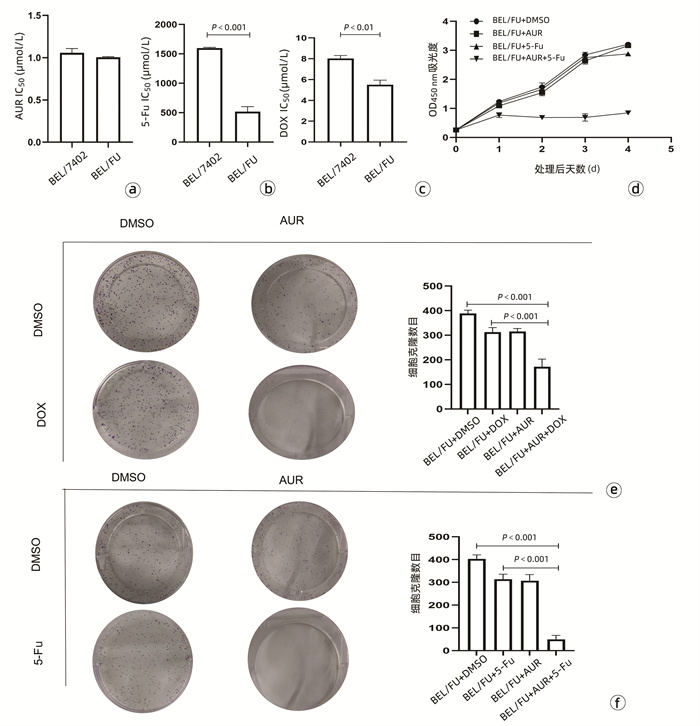
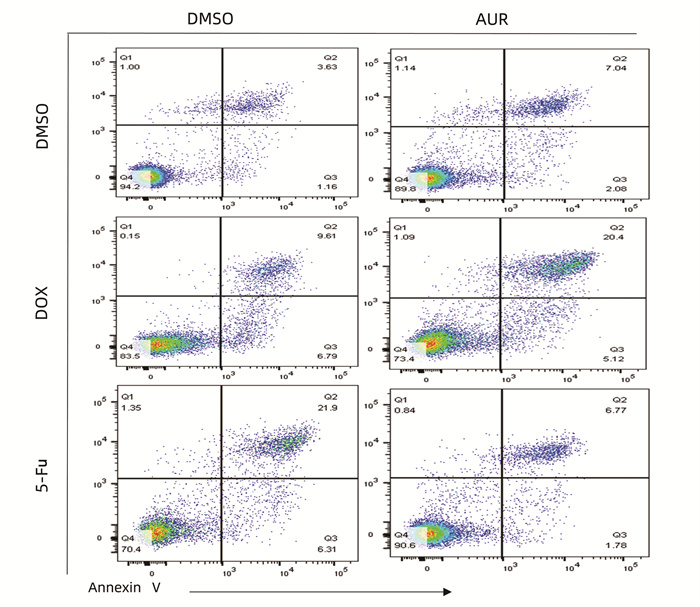
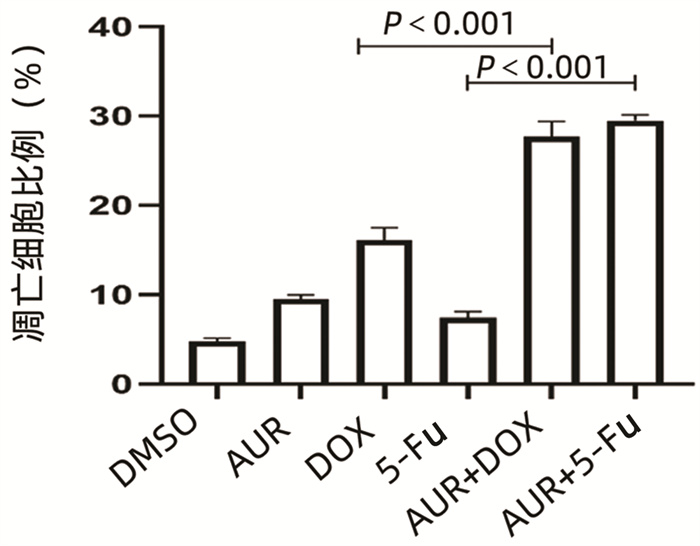
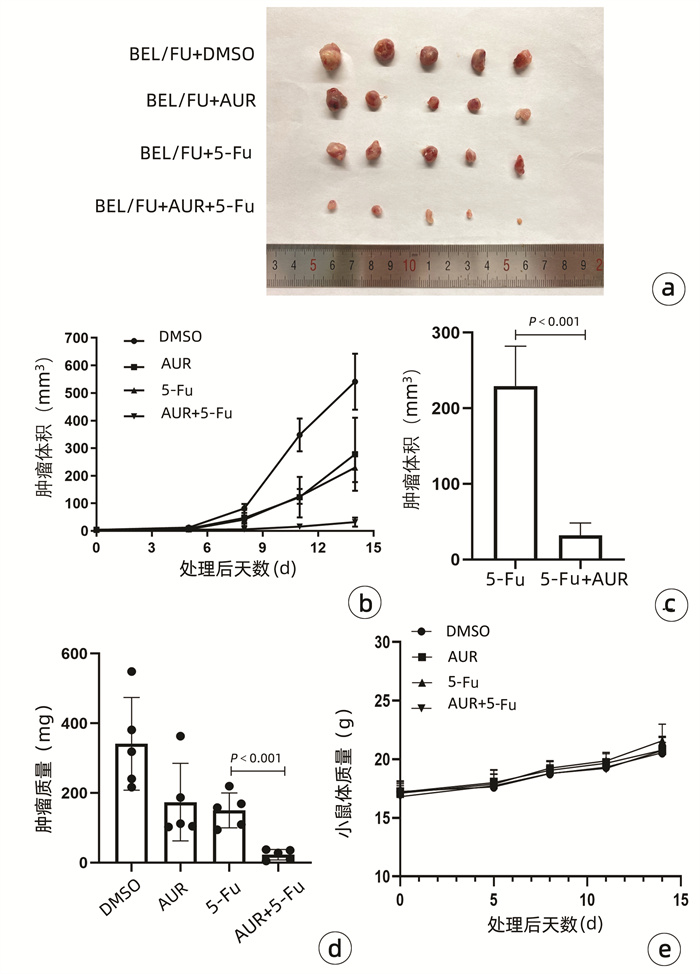
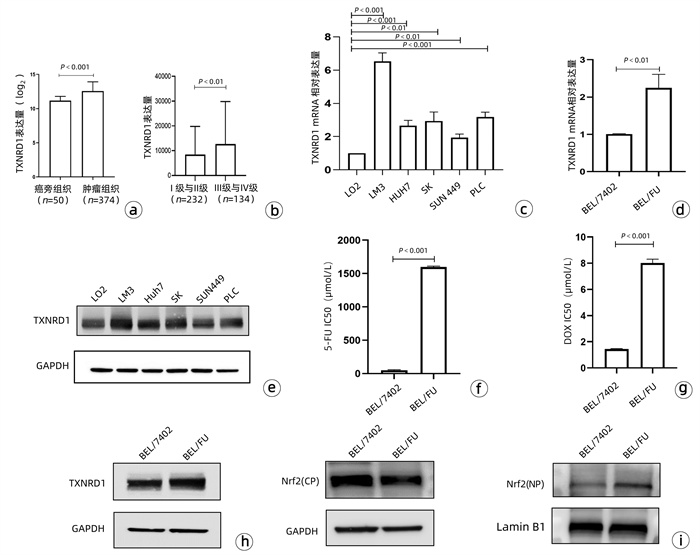
目的 耐药性是肝细胞癌(HCC)化疗失败的主要原因,硫氧还蛋白还原酶1(TXNRD1)作为活性氧(ROS)代谢的主要影响因素,已被证明与HCC患者的不良预后有关。本研究探究TXNRD1在HCC多药耐药机制中的作用。 方法 选取BEL-7402细胞系的BEL/FU细胞作为多重耐药细胞株。siRNA用来干预细胞TXNRD1的表达;实时定量PCR和蛋白质印迹检测TXNRD1的表达;CCK-8法检测和流式细胞术检测用来评估TXNRD1对肝癌细胞ROS蓄积、5-氟尿嘧啶(5-Fu)和多柔比星(DOX)耐药性以及体外凋亡的影响;异种肿瘤移植模型的构建用来研究金诺芬(AUR)在体内对细胞耐药性的影响。计量资料两组间比较采用独立样本t检验。 结果 BEL/FU作为多重耐药HCC细胞系表现出TXNRD1 mRNA与蛋白水平的高表达(P值均<0.05)。AUR作为TXNRD1的抑制剂,联合化疗药物5-FU及DOX处理与单一化疗药物处理相比,细胞克隆集落形成数目明显减少(P值均<0.01),凋亡比例明显增加(P值均<0.001)。N-乙酰半胱氨酸(NAC)作为ROS的清除剂,可以明显削弱siRNA敲低TXNRD1表达对BEL/FU细胞耐药性的影响,应用NAC可以有效降低干扰后细胞的凋亡比例(P值均<0.001)。动物实验也证实,5-Fu与AUR联合治疗相对于单一5-Fu治疗,裸鼠的肿瘤质量更轻(P<0.001),体积更小(P<0.001)。 结论 TXNRD1在HCC耐药中扮演重要角色,抑制其在细胞水平作用可有效改善耐药性,AUR作为TXNRD1抑制剂在HCC综合治疗中也具备极大的应用前景。 Abstract:Objective Drug resistance is the main cause of chemotherapy failure in hepatocellular carcinoma (HCC), and thioredoxin reductase 1 (TXNRD1), as a major influencing factor for reactive oxygen species (ROS) metabolism, has been proven to be associated with the poor prognosis of patients with HCC. This study aims to explore the role of TXNRD1 in the mechanism of multidrug resistance in HCC. Methods BEL/FU cells in BEL-7402 cell line were selected as the multidrug-resistant cell line. The siRNA was used for the intervention of TXNRD1 expression; quantitative real-time PCR and Western blotting were used to measure the expression of TXNRD1; CCK-8 assay and flow cytometry were used to evaluate the effect of TXNRD1 on hepatocyte ROS accumulation, resistance to 5-fluorouracil (5-Fu) and doxorubicin (DOX), and apoptosis in vitro; a xenograft tumor model was established to investigate the effect of auranofin (AUR) on drug resistance in vivo. The two-independent-samples t test was used for comparison of continuous data between two groups. Results As a multidrug-resistant HCC cell line, BEL/Fu showed high mRNA and protein expression levels of TXNRD1 (both P < 0.05). Compared with 5-Fu or DOX treatment alone, the TXNRD1 inhibitor AUR combined with 5-Fu or DOX had had a significant reduction in the number of colony formation (P < 0.01) and a significant increase in apoptosis ratio (P < 0.001). The ROS scavenger N-acetylcysteine (NAC) significantly weakened the effect of TXNRD1 knockdown by siRNA on the drug resistance of BEL/Fu cells, and the application of NAC effectively reduced the apoptosis ratio of cells after siRNA interference (P < 0.001). Animal experiments also confirmed that compared with the nude mice treated with 5-Fu alone, the nude mice treated with 5-Fu and AUR had a significantly lower tumor mass (P < 0.001) and a significantly smaller tumor volume (P < 0.001). Conclusion TXNRD1 plays an important role in the drug resistance of HCC, and inhibition of its level in cells can effectively improve drug resistance. As a TXNRD1 inhibitor, AUR has great application prospects in the multimodality therapy for HCC. -
表 1 引物序列
基因名 引物 序列(5′→ 3′) TXNRD1 正向 ATGGTGCTTGTGGCCTTTCT 反向 GCCCACAACACGTTCATTGTC TXNRD2 正向 GGCTTCGACCAGCAAATGTC 反向 CACAGGACGGTGTCAAAGGT TXNRD3 正向 GCTCCTTCAGGAAGATTTGGC 反向 GGGACAACAAAGTCTAGCACCA GAPDH 正向 GGAGCGAGATCCCTCCAAAAT 反向 GGCTGTTGTCATACTTCTCATGG -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] CHEN Z, XIE H, HU M, et al. Recent progress in treatment of hepatocellular carcinoma[J]. Am J Cancer Res, 2020, 10(9): 2993-3036. [3] SZAKÁCS G, PATERSON JK, LUDWIG JA, et al. Targeting multidrug resistance in cancer[J]. Nat Rev Drug Discov, 2006, 5(3): 219-234. DOI: 10.1038/nrd1984. [4] BUKOWSKI K, KCIUK M, KONTEK R. Mechanisms of multidrug resistance in cancer chemotherapy[J]. Int J Mol Sci, 2020, 21(9): 3233. DOI: 10.3390/ijms21093233. [5] KLAUNIG JE. Oxidative stress and cancer[J]. Curr Pharm Des, 2018, 24(40): 4771-4778. DOI: 10.2174/1381612825666190215121712. [6] KUDRYAVTSEVA AV, KRASNOV GS, DMITRIEV AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer[J]. Oncotarget, 2016, 7(29): 44879-44905. DOI: 10.18632/oncotarget.9821. [7] JAKUBCZYK K, DEC K, KAŁDUŃSKA J, et al. Reactive oxygen species-sources, functions, oxidative damage[J]. Pol Merkur Lekarski, 2020, 48(284): 124-127. [8] AKIN-BALI DF, AL-KHAFAJI K, AKTAS SH, et al. Bioinformatic and computational analysis for predominant mutations of the Nrf2/Keap1 complex in pediatric leukemia[J]. J Biomol Struct Dyn, 2021, 39(12): 4290-4303. DOI: 10.1080/07391102.2020.1775702. [9] GAO Q, ZHANG G, ZHENG Y, et al. SLC27A5 deficiency activates NRF2/TXNRD1 pathway by increased lipid peroxidation in HCC[J]. Cell Death Differ, 2020, 27(3): 1086-1104. DOI: 10.1038/s41418-019-0399-1. [10] HUANG S, ZHU X, KE Y, et al. LncRNA FTX inhibition restrains osteosarcoma proliferation and migration via modulating miR-320a/TXNRD1[J]. Cancer Biol Ther, 2020, 21(4): 379-387. DOI: 10.1080/15384047.2019.1702405. [11] PARK N, CHUN YJ. Auranofin promotes mitochondrial apoptosis by inducing annexin A5 expression and translocation in human prostate cancer cells[J]. J Toxicol Environ Health A, 2014, 77(22-24): 1467-1476. DOI: 10.1080/15287394.2014.955834. [12] FU B, MENG W, ZENG X, et al. TXNRD1 is an unfavorable prognostic factor for patients with hepatocellular carcinoma[J]. Biomed Res Int, 2017, 2017: 4698167. DOI: 10.1155/2017/4698167. [13] PRASAD S, GUPTA SC, TYAGI AK. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals[J]. Cancer Lett, 2017, 387: 95-105. DOI: 10.1016/j.canlet.2016.03.042. [14] LIU N, GUO Z, XIA X, et al. Auranofin lethality to prostate cancer includes inhibition of proteasomal deubiquitinases and disrupted androgen receptor signaling[J]. Eur J Pharmacol, 2019, 846: 1-11. DOI: 10.1016/j.ejphar.2019.01.004. [15] LIOU GY, STORZ P. Reactive oxygen species in cancer[J]. Free Radic Res, 2010, 44(5): 479-496. DOI: 10.3109/10715761003667554. [16] SEIFRIED HE, ANDERSON DE, SORKIN BC, et al. Free radicals: The pros and cons of antioxidants. Executive summary report[J]. J Nutr, 2004, 134(11): 3143S-3163S. DOI: 10.1093/jn/134.11.3143S. [17] SUN C, ZHANG H, MA XF, et al. Isoliquiritigenin enhances radiosensitivity of HepG2 cells via disturbance of redox status[J]. Cell Biochem Biophys, 2013, 65(3): 433-444. DOI: 10.1007/s12013-012-9447-x. [18] LING S, LI J, SHAN Q, et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway[J]. Mol Oncol, 2017, 11(6): 682-695. DOI: 10.1002/1878-0261.12067. [19] ZHOU Y, WANG Y, ZHOU W, et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway[J]. Cancer Cell Int, 2019, 19: 179. DOI: 10.1186/s12935-019-0898-7. [20] KIM NH, PARK HJ, OH MK, et al. Antiproliferative effect of gold(I) compound auranofin through inhibition of STAT3 and telomerase activity in MDA-MB 231 human breast cancer cells[J]. BMB Rep, 2013, 46(1): 59-64. DOI: 10.5483/bmbrep.2013.46.1.123. [21] HWANG-BO H, JEONG JW, HAN MH, et al. Auranofin, an inhibitor of thioredoxin reductase, induces apoptosis in hepatocellular carcinoma Hep3B cells by generation of reactive oxygen species[J]. Gen Physiol Biophys, 2017, 36(2): 117-128. DOI: 10.4149/gpb_2016043. [22] LIU JJ, LIU Q, WEI HL, et al. Inhibition of thioredoxin reductase by auranofin induces apoptosis in adriamycin-resistant human K562 chronic myeloid leukemia cells[J]. Pharmazie, 2011, 66(6): 440-444. [23] ZHENG Y, LIU Y, ZHAO S, et al. Large-scale analysis reveals a novel risk score to predict overall survival in hepatocellular carcinoma[J]. Cancer Manag Res, 2018, 10: 6079-6096. DOI: 10.2147/CMAR.S181396. [24] CARLSON BA, YOO MH, TOBE R, et al. Thioredoxin reductase 1 protects against chemically induced hepatocarcinogenesis via control of cellular redox homeostasis[J]. Carcinogenesis, 2012, 33(9): 1806-1813. DOI: 10.1093/carcin/bgs230. [25] OZBEN T. Antioxidant supplementation on cancer risk and during cancer therapy: An update[J]. Curr Top Med Chem, 2015, 15(2): 170-178. DOI: 10.2174/1568026615666141209160918 [26] CALAF GM, AGUAYO F, SERGI CM, et al. Antioxidants and cancer: Theories, techniques, and trials in preventing cancer[J]. Oxid Med Cell Longev, 2018, 2018: 5363064. DOI: 10.1155/2018/5363064. [27] KLEIN EA, THOMPSON IM Jr, TANGEN CM, et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E Cancer Prevention Trial (SELECT)[J]. JAMA, 2011, 306(14): 1549-1556. DOI: 10.1001/jama.2011.1437. [28] ARNÉR E. Targeting the selenoprotein thioredoxin reductase 1 for anticancer therapy[J]. Adv Cancer Res, 2017, 136: 139-151. DOI: 10.1016/bs.acr.2017.07.005. -



 PDF下载 ( 8489 KB)
PDF下载 ( 8489 KB)

 下载:
下载: