Hepatocyte-specific TM6SF2 knockout aggravates hepatic steatosis in mice with nonalcoholic fatty liver disease
-
摘要:
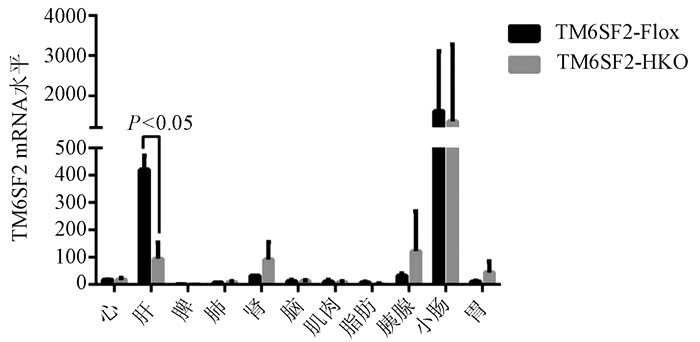
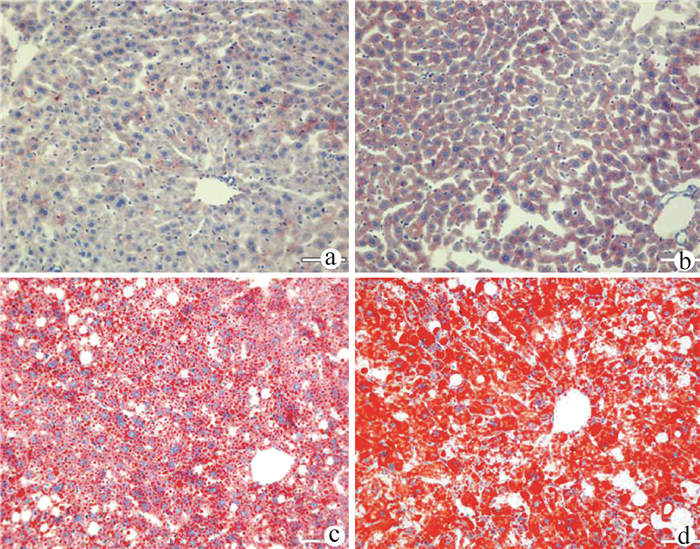
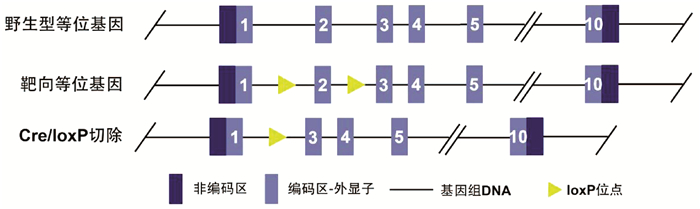
目的 构建TM6SF2肝细胞特异性敲除的小鼠模型,研究TM6SF2在非酒精性脂肪性肝病(NAFLD)发病中的作用。 方法 利用CRISPR/Cas9技术和Cre/LoxP策略构建稳定的TM6SF2肝细胞特异性敲除的小鼠模型。给予TM6SF2肝细胞特异性敲除小鼠和对照小鼠普通饮食或高脂饮食喂养16周,检测小鼠体质量、肝质量等一般情况,空腹血糖、胰岛素等糖代谢相关指标,以及血浆甘油三酯、胆固醇、肝脏甘油三酯等脂代谢相关的指标。符合正态分布的计量资料两组之间比较使用t检验。 结果 在高脂饮食条件下,TM6SF2肝细胞特异性敲除小鼠的肝质量[(2.235±0.175)g vs (1.258±0.106)g,t=4.789, P<0.01]和肝指数(4.970%± 0.298% vs 3.210%±0.094%,t=5.630, P<0.01)明显高于对照小鼠。同时,肝细胞TM6SF2基因缺失也会加重高脂饮食诱导的ALT水平异常[(62.517±1.526)U/L vs (25.991±5.947)U/L, t=5.949, P<0.01]。相较于对照组,在普通饮食和高脂饮食条件下,TM6SF2敲除小鼠血浆胰岛素水平升高[(37.203±0.836)mIU/L vs(34.835±0.426)mIU/L,t=2.520, P=0.025;(41.093±1.226)mIU/L vs(35.817 ±0.500)mIU/L,t=3.985, P=0.007],其他与糖代谢相关指标差异均无统计学意义(P值均>0.05)。在高脂饮食条件下,TM6SF2肝细胞特异性敲除小鼠血浆甘油三酯和胆固醇水平较对照组差异无统计学意义(P值均>0.05),而肝脏内甘油三酯水平较对照组升高[(23.969±0.978)mg/g vs(18.229±1.633) mg/g,t=3.015, P=0.024]。 结论 肝细胞特异性敲除TM6SF2可加重NAFLD小鼠肝脏脂质蓄积和肝损伤。 -
关键词:
- 非酒精性脂肪性肝病 /
- TM6SF2 /
- CRISPR/Cas9
Abstract:Objective To establish a mouse model of hepatocyte-specific TM6SF2 knockout, and to investigate the role of TM6SF2 in the development of nonalcoholic fatty liver disease (NAFLD). Methods The CRISPR/Cas9 technique and the Cre/LoxP strategy were used to establish a stable mouse model of hepatocyte-specific TM6SF2 knockout. The mice with hepatocyte-specific TM6SF2 knockout and the control mice were given a normal diet or a high-fat diet (HFD) for 16 weeks, and related indices were measured, including general status (body weight and liver weight), glucose metabolic indices (fasting blood glucose and insulin), and lipid metabolism (plasma triglyceride, cholesterol, and liver triglyceride). The t-test was used for comparison of normally distributed continuous data between two groups. Results Under the condition of HFD, compared with the control mice, the mice with hepatocyte-specific TM6SF2 knockout had significantly higher liver weight (2.235±0.175 g vs 1.258±0.106 g, t=4.789, P < 0.01) and liver index (4.970%±0.298% vs 3.210%±0.094%, t=5.630, P < 0.01), and the loss of the TM6SF2 gene in hepatocytes aggravated the abnormal level of alanine aminotransferase induced by HFD (62.517±1.526 U/L vs 25.991±5.947 U/L, t=5.949, P < 0.01). Compared with the control mice under the condition of normal diet or HFD, the mice with TM6SF2 knockout had a significant increase in plasma insulin level (normal diet: 37.203±0.836 mIU/L vs 34.835±0.426 mIU/L, t=2.520, P=0.025; HFD: 41.093±1.226 mIU/L vs 35.817±0.500 mIU/L, t=3.985, P=0.007), while there were no significant differences in the other indices associated with glucose metabolism (all P > 0.05). Under the condition of HFD, there were no significant differences in the levels of plasma triglyceride and cholesterol between the mice with hepatocyte-specific TM6SF2 knockout and the control group (P > 0.05), while the mice with hepatocyte-specific TM6SF2 knockout had a significant increase in the level of liver triglyceride compared with the control mice (23.969±0.978 mg/g vs 18.229±1.633 mg/g, t=3.015, P=0.024). Conclusion Hepatocyte-specific knockout of TM6SF2 can aggravate liver lipid accumulation and liver injury in mice with NAFLD. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- TM6SF2 /
- CRISPR/Cas9
-
表 1 基因型鉴定对应的引物序列
引物 序列(5′→3′) TM6SF2-F CAGTGTCAAGATTAGTGTTGGC TM6SF2-R CACCGAAGTTATACAGTCTGAT Cre-F ATATCTCACGTACTGACGGTGG Cre-R CCTTAGCGCCGTAAATCAATC 表 2 TM6SF2-HKO对小鼠肝质量、肝酶等指标影响
指标 TM6SF2-Flox-CD(n=8) TM6SF2-HKO-CD(n=8) t值 P值 TM6SF2-Flox- HFD(n=4) TM6SF2-HKO- HFD (n=4) t值 P值 体质量(g) 25.996±0.483 27.150±0.545 1.585 0.135 39.088±2.436 44.830±0.937 2.200 0.070 肝质量(g) 1.115±0.032 1.117±0.027 0.417 0.967 1.258±0.106 2.235±0.175 4.789 0.003 肝指数(%) 4.284±0.076 4.111±0.050 1.900 0.078 3.210±0.094 4.970±0.298 5.630 0.001 血浆ALT (U/L) 15.014±2.670 10.377±1.433 1.530 0.148 25.991±5.947 62.517±1.526 5.949 0.001 血浆AST (U/L) 30.343±1.723 26.525±1.649 1.601 0.132 26.645±2.576 33.485±3.075 1.705 0.139 注:CD,普通饮食;HFD,高脂饮食。 表 3 肝细胞TM6SF2缺失对小鼠糖代谢影响
指标 TM6SF2-Flox- CD(n=8) TM6SF2-HKO- CD(n=8) t值 P值 TM6SF2-Flox- HFD(n=4) TM6SF2-HKO- HFD(n=4) t值 P值 空腹血糖(mmol/L) 7.495±0.571 7.347±0.403 0.211 0.836 9.877±1.017 9.489±0.621 0.325 0.756 胰岛素(mIU/L) 34.835±0.426 37.203±0.836 2.520 0.025 35.817±0.500 41.093±1.226 3.985 0.007 OGTT-0 min-血糖(mmol/L) 6.425±0.373 5.875±0.653 0.731 0.477 9.600±0.402 10.000±1.042 0.358 0.732 OGTT-30 min-血糖(mmol/L) 15.200±0.645 14.980±0.666 0.243 0.812 21.830±2.862 24.38±1.518 0.787 0.461 OGTT-60 min-血糖(mmol/L) 9.275±0.609 9.263±0.368 0.018 0.986 22.080±3.726 20.050±1.599 0.500 0.635 OGTT-120 min-血糖(mmol/L) 5.900±0.403 6.438±0.455 0.884 0.392 15.080±1.805 12.630±1.278 1.108 0.310 OGTT曲线下面积(mol·min/L) 1.147±0.043 1.147±0.053 0.008 0.994 2.162±0.141 2.244±0.308 0.243 0.817 胰岛素抵抗指数(mmol·mIU/L2) 11.608±0.921 12.134±0.689 0.458 0.654 15.694±1.528 17.332±1.227 0.836 0.435 表 4 TM6SF2-HKO对小鼠脂代谢影响
指标 TM6SF2-Flox- CD(n=8) TM6SF2-HKO- CD(n=8) t值 P值 TM6SF2-Flox- HFD(n=4) TM6SF2-HKO- HFD(n=4) t值 P值 血浆TC(mmol/L) 1.182±0.235 1.159±0.191 0.772 0.940 2.203±0.661 2.337±0.681 0.940 0.383 血浆TG(mmol/L) 0.874±0.048 0.724±0.058 2.003 0.065 1.046±0.213 0.914±0.010 0.563 0.594 肝组织TG(mg/g) 11.935±0.597 13.240±0.658 1.467 0.164 18.229±1.633 23.969±0.978 3.015 0.024 -
[1] COBBINA E, AKHLAGHI F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters[J]. Drug Metab Rev, 2017, 49(2): 197-211. DOI: 10.1080/03602532.2017.1293683. [2] WU TF, LIAO XH, ZHONG BH. Epidemiology of nonalcoholic fatty liver disease in some regions of China[J]. J Clin Hepatol, 2020, 36(6): 1370-1373. DOI: 10.3969/j.issn.1001-5256.2020.06.039.吴挺丰, 廖献花, 钟碧慧. 中国部分地区非酒精性脂肪肝病的流行情况[J]. 临床肝胆病杂志, 2020, 36(6): 1370-1373. DOI: 10.3969/j.issn.1001-5256.2020.06.039. [3] YOUNOSSI Z, ANSTEE QM, MARIETTI M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(1): 11-20. DOI: 10.1038/nrgastro.2017.109. [4] BYRNE CD, TARGHER G. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease: Is universal screening appropriate?[J]. Diabetologia, 2016, 59(6): 1141-1144. DOI: 10.1007/s00125-016-3910-y. [5] LEUNG C, RIVERA L, FURNESS JB, et al. The role of the gut microbiota in NAFLD[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(7): 412-425. DOI: 10.1038/nrgastro.2016.85. [6] KOZLITINA J, SMAGRIS E, STENDER S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease[J]. Nat Genet, 2014, 46(4): 352-356. DOI: 10.1038/ng.2901. [7] SOOKOIAN S, CASTAÑO GO, SCIAN R, et al. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity[J]. Hepatology, 2015, 61(2): 515-525. DOI: 10.1002/hep.27556. [8] KRAWCZYK M, RAU M, SCHATTENBERG JM, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: A multicenter biopsy-based study[J]. J Lipid Res, 2017, 58(1): 247-255. DOI: 10.1194/jlr.P067454. [9] GOFFREDO M, CAPRIO S, FELDSTEIN AE, et al. Role of TM6SF2 rs58542926 in the pathogenesis of nonalcoholic pediatric fatty liver disease: A multiethnic study[J]. Hepatology, 2016, 63(1): 117-125. DOI: 10.1002/hep.28283. [10] ANDRIKOPOULOS S, BLAIR AR, DELUCA N, et al. Evaluating the glucose tolerance test in mice[J]. Am J Physiol Endocrinol Metab, 2008, 295(6): E1323-1332. DOI: 10.1152/ajpendo.90617.2008. [11] MA Y, YU L, PAN S, et al. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre-loxP-mediated lineage tracing[J]. FEBS J, 2017, 284(19): 3262-3277. DOI: 10.1111/febs.14188. [12] FAN Y, LU H, GUO Y, et al. Hepatic Transmembrane 6 superfamily member 2 regulates cholesterol metabolism in mice[J]. Gastroenterology, 2016, 150(5): 1208-1218. DOI: 10.1053/j.gastro.2016.01.005. [13] GUPTA D, BHATTACHARJEE O, MANDAL D, et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing[J]. Life Sci, 2019, 232: 116636. DOI: 10.1016/j.lfs.2019.116636. [14] HRYHOROWICZ M, LIPIŃSKI D, ZEYLAND J, et al. CRISPR/Cas9 immune system as a tool for genome engineering[J]. Arch Immunol Ther Exp (Warsz), 2017, 65(3): 233-240. DOI: 10.1007/s00005-016-0427-5. [15] YANG J, ZHU D, JU B, et al. Hepatoprotective effects of Gentianella turkestanerum extracts on acute liver injury induced by carbon tetrachloride in mice[J]. Am J Transl Res, 2017, 9(2): 569-579. [16] HWANG YP, CHOI JH, JEONG HG. Protective effect of the Aralia continentalis root extract against carbon tetrachloride-induced hepatotoxicity in mice[J]. Food Chem Toxicol, 2009, 47(1): 75-81. DOI: 10.1016/j.fct.2008.10.011. [17] Diagnosis and Treatment Center of Hepatology of South China Alliance of TCM, National Administration of Traditional Chinese Medicine. Diagnosis and treatment scheme of Ganpi (non-alcoholic steatohepatitis)[J/CD]. Chin J Liver Dis: Electronic Edition, 2021, 13(1): 1-9. DOI: 10.3969/j.issn.1674-7380.2021.01.001国家中医药管理局华南区中医肝病诊疗中心联盟. 肝癖(非酒精性脂肪性肝炎)诊疗方案[J/CD]. 中国肝脏病杂志(电子版), 2021, 13(1): 1-9. DOI: 10.3969/j.issn.1674-7380.2021.01.001 [18] PETERSEN MC, VATNER DF, SHULMAN GI. Regulation of hepatic glucose metabolism in health and disease[J]. Nat Rev Endocrinol, 2017, 13(10): 572-587. DOI: 10.1038/nrendo.2017.80. [19] WATT MJ, MIOTTO PM, de NARDO W, et al. The liver as an endocrine organ-linking nafld and insulin resistance[J]. Endocr Rev, 2019, 40(5): 1367-1393. DOI: 10.1210/er.2019-00034. -



 PDF下载 ( 3743 KB)
PDF下载 ( 3743 KB)

 下载:
下载: