昼夜节律及其相关基因与非酒精性脂肪性肝病的关系
DOI: 10.3969/j.issn.1001-5256.2021.09.045
利益冲突声明: 所有作者均声明不存在利益冲突。
作者贡献声明: 赵晨露负责撰写论文;赵文霞负责指导撰写文章、修改论文。
Association of circadian rhythm and related genes with nonalcoholic fatty liver disease
-
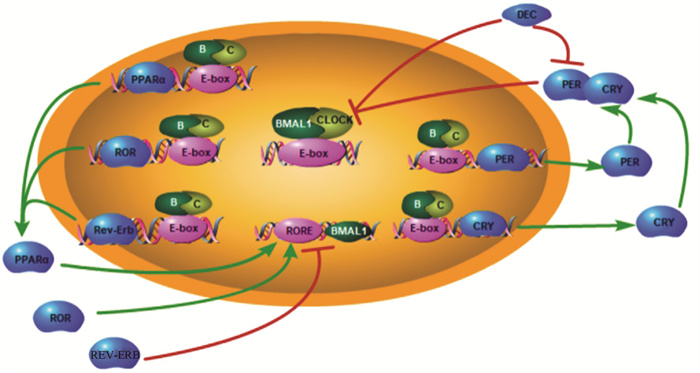
摘要: 近年来昼夜节律与代谢性疾病的关系逐渐受到人们重视。大量临床研究表明, 经常熬夜、轮班的人群非酒精性脂肪性肝病的发生风险明显高于作息规律的人群。基于现有数据, 重点从脂质代谢、葡萄糖代谢、肠道菌群、氧化应激、内质网应激五个方面总结了昼夜节律基因在非酒精性脂肪性肝病中的作用机制。Abstract: The association between circadian rhythm and metabolic diseases has attracted more and more attention in recent years. A large number of clinical studies have shown that people who often stay up late or work in shifts have a significantly higher risk of nonalcoholic fatty liver disease than those with regular work and rest. Based on current research findings, this article reviews the mechanism of action of circadian rhythm genes in nonalcoholic fatty liver disease from the five aspects of lipid metabolism, glucose metabolism, intestinal flora, oxidative stress, and endoplasmic reticulum stress.
-
Key words:
- Circadian Rhythm /
- Gene /
- Non-alcoholic Fatty Liver Disease
-
表 1 不同部位昼夜节律基因BMAL1敲除或沉默对糖代谢的影响
作者(年份) 基因敲除/沉默 部位 表型 叶绿, 等(2020) Bmal1-沉默 胰腺 ROS、Nrf2表达增加, GSIS、CAT、GSH-Px水平升高 叶绿, 等(2020) Bmal1-沉默 胰腺 ROS和细胞凋亡增加, Sirt1 mRNA表达减少 Curtis AM, 等(2007) Bmal1-/- 全身 血压和心率昼夜节律改变 Anea CB, 等(2009) Bmal1-/- 全身 胆固醇和甘油三酯升高, 血管重构受损, 血管损伤和内皮功能障碍 Marcheva B, 等(2010) Bmal1-/- 全身 胰岛素抵抗、肥胖, 葡萄糖耐受不良, 胰岛素分泌减少 Shi S-q, 等(2013) Bmal1-/- 全身 葡萄糖耐受不良, 胰岛素分泌减少 Shimba S, 等(2011) Bmal1-/- 全身 游离脂肪酸、甘油三酯、胆固醇水平增高, 脂肪组织、肝脏、肌肉组织脂肪沉积 Sadacca LA, 等(2011) Bmal1-/- 胰腺 胰岛素分泌改变 Paschos GK, 等(2012) Bmal1-/- 脂肪组织 进食行为改变、肥胖 Xie Z, 等(2015) Bmal1-/- 平滑肌 胰岛细胞大小和增殖受损, 血压节律丧失 Harfmann BD, 等(2016) Bmal1-/- 骨骼肌 肌肉中的葡萄糖代谢受损和紊乱 Chaix A, 等(2019) Bmal1-/- 肝脏 血清甘油三酯升高, 肝内脂肪沉积, 循环葡萄糖清除受损 Lamia KA, 等(2008) Bmal1-/- 肝脏 血清甘油三酯升高, 肝内脂肪沉积, 循环葡萄糖清除受损 -
[1] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014. e1. DOI: 10.1053/j.gastro.2019.11.312. [2] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24(7): 908-922. DOI: 10.1038/s41591-018-0104-9. [3] GNOCCHI D, CUSTODERO C, SABBÀ C, et al. Circadian rhythms: A possible new player in non-alcoholic fatty liver disease pathophysiology[J]. J Mol Med (Berl), 2019, 97(6): 741-759. DOI: 10.1007/s00109-019-01780-2. [4] MAZZOCCOLI G, de COSMO S, MAZZA T. The biological clock: A pivotal hub in non-alcoholic fatty liver disease pathogenesis[J]. Front Physiol, 2018, 9: 193. DOI: 10.3389/fphys.2018.00193. [5] REINKE H, ASHER G. Circadian clock control of liver metabolic functions[J]. Gastroenterology, 2016, 150(3): 574-580. DOI: 10.1053/j.gastro.2015.11.043. [6] SHETTY A, HSU JW, MANKA PP, et al. Role of the circadian clock in the metabolic syndrome and nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2018, 63(12): 3187-3206. DOI: 10.1007/s10620-018-5242-x. [7] BERSON DM, DUNN FA, TAKAO M. Phototransduction by retinal ganglion cells that set the circadian clock[J]. Science, 2002, 295(5557): 1070-1073. DOI: 10.1126/science.1067262. [8] BROWN SA, KOWALSKA E, DALLMANN R. (Re)inventing the circadian feedback loop[J]. Dev Cell, 2012, 22(3): 477-487. DOI: 10.1016/j.devcel.2012.02.007. [9] CANAPLE L, RAMBAUD J, DKHISSI-BENYAHYA O, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock[J]. Mol Endocrinol, 2006, 20(8): 1715-1727. DOI: 10.1210/me.2006-0052. [10] SATO F, KOHSAKA A, BHAWAL UK, et al. Potential roles of DEC and BMAL1 genes in interconnecting circadian clock and energy metabolism[J]. Int J Mol Sci, 2018, 19(3): 781. DOI: 10.3390/ijms19030781. [11] YOSHITANE H, ASANO Y, SAGAMI A, et al. Functional D-box sequences reset the circadian clock and drive mRNA rhythms[J]. Commun Biol, 2019, 2: 300. DOI: 10.1038/s42003-019-0522-3. [12] SHAN Z, LI Y, ZONG G, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses[J]. BMJ, 2018, 363: k4641. DOI: 10.1136/bmj.k4641. [13] BUXTON OM, CAIN SW, O'CONNOR SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption[J]. Sci Transl Med, 2012, 4(129): 129ra43. DOI: 10.1126/scitranslmed.3003200. [14] ADAFER R, MESSAADI W, MEDDAHI M, et al. Food timing, circadian rhythm and chrononutrition: A systematic review of time-restricted eating's effects on human health[J]. Nutrients, 2020, 12(12): 3770. DOI: 10.3390/nu12123770. [15] DERBOUZ ROUIBATE A, BENHAFRI N, OUALI-HASSENAOUI S, et al. The Light/Dark cycle disruption affects hepatic function both in metabolic parameters and tissue structure in a nocturnal desert rodent: Gerbillus tarabuli[J]. Folia Histochem Cytobiol, 2020, 58(3): 182-197. DOI: 10.5603/FHC.a2020.0021. [16] YAMAMURO D, TAKAHASHI M, NAGASHIMA S, et al. Peripheral circadian rhythms in the liver and white adipose tissue of mice are attenuated by constant light and restored by time-restricted feeding[J]. PLoS One, 2020, 15(6): e0234439. DOI: 10.1371/journal.pone.0234439. [17] ZHANG R, LAHENS NF, BALLANCE HI, et al. A circadian gene expression atlas in mammals: Implications for biology and medicine[J]. Proc Natl Acad Sci U S A, 2014, 111(45): 16219-16224. DOI: 10.1073/pnas.1408886111. [18] DERBOUZ ROUIBATE A, BENHAFRI N, OUALI-HASSENAOUI S, et al. The Light/Dark cycle disruption affects hepatic function both in metabolic parameters and tissue structure in a nocturnal desert rodent: Gerbillus tarabuli[J]. Folia Histochem Cytobiol, 2020, 58(3): 182-197. DOI: 10.5603/FHC.a2020.0021. [19] TUREK FW, JOSHU C, KOHSAKA A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice[J]. Science, 2005, 308(5724): 1043-1045. DOI: 10.1126/science.1108750. [20] LANDGRAF D, NEUMANN AM, OSTER H. Circadian clock-gastrointestinal peptide interaction in peripheral tissues and the brain[J]. Best Pract Res Clin Endocrinol Metab, 2017, 31(6): 561-571. DOI: 10.1016/j.beem.2017.10.007. [21] PAN X, BRADFIELD CA, HUSSAIN MM. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis[J]. Nat Commun, 2016, 7: 13011. DOI: 10.1038/ncomms13011. [22] PASCHOS GK, IBRAHIM S, SONG WL, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl[J]. Nat Med, 2012, 18(12): 1768-1777. DOI: 10.1038/nm.2979. [23] GOOLEY JJ. Circadian regulation of lipid metabolism[J]. Proc Nutr Soc, 2016, 75(4): 440-450. DOI: 10.1017/S0029665116000288. [24] HUNTER AL, PELEKANOU CE, ADAMSON A, et al. Nuclear receptor REVERBα is a state-dependent regulator of liver energy metabolism[J]. Proc Natl Acad Sci U S A, 2020, 117(41): 25869-25879. DOI: 10.1073/pnas.2005330117. [25] MAZZOCCOLI G, de COSMO S, MAZZA T. The biological clock: A pivotal hub in non-alcoholic fatty liver disease pathogenesis[J]. Front Physiol, 2018, 9: 193. DOI: 10.3389/fphys.2018.00193. [26] LEE J, LEE S, CHUNG S, et al. Identification of a novel circadian clock modulator controlling BMAL1 expression through a ROR/REV-ERB-response element-dependent mechanism[J]. Biochem Biophys Res Commun, 2016, 469(3): 580-586. DOI: 10.1016/j.bbrc.2015.12.030. [27] TOKAT B, KANCA-DEMIRCI D, GUL N, et al. Determination of genetic changes of Rev-erb beta and Rev-erb alpha genes in Type 2 diabetes mellitus by next-generation sequencing[J]. Gene, 2020, 763: 145058. DOI: 10.1016/j.gene.2020.145058. [28] MONNIER C, AUCLAIR M, LE CAM G, et al. The nuclear retinoid-related orphan receptor RORα controls circadian thermogenic programming in white fat depots[J]. Physiol Rep, 2018, 6(8): e13678. DOI: 10.14814/phy2.13678. [29] HAN YH, KIM HJ, NA H, et al. RORα Induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis[J]. Cell Rep, 2017, 20(1): 124-135. DOI: 10.1016/j.celrep.2017.06.017. [30] SHI D, CHEN J, WANG J, et al. Circadian clock genes in the metabolism of non-alcoholic fatty liver disease[J]. Front Physiol, 2019, 10: 423. DOI: 10.3389/fphys.2019.00423. [31] AGGARWAL A, COSTA MJ, RIVERO-GUTIÉRREZ B, et al. The circadian clock regulates adipogenesis by a Per3 crosstalk pathway to Klf15[J]. Cell Rep, 2017, 21(9): 2367-2375. DOI: 10.1016/j.celrep.2017.11.004. [32] OOSTERMAN JE, WOPEREIS S, KALSBEEK A. The circadian clock, shift work, and tissue-specific insulin resistance[J]. Endocrinology, 2020, 161(12): bqaa180. DOI: 10.1210/endocr/bqaa180. [33] PETRENKO V, GANDASI NR, SAGE D, et al. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis[J]. Proc Natl Acad Sci U S A, 2020, 117(5): 2484-2495. DOI: 10.1073/pnas.1916539117 [34] SHIMBA S, OGAWA T, HITOSUGI S, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation[J]. PLoS One, 2011, 6(9): e25231. DOI: 10.1371/journal.pone.0025231. [35] XIONG X, LIN Y, LEE J, et al. Chronic circadian shift leads to adipose tissue inflammation and fibrosis[J]. Mol Cell Endocrinol, 2021, 521: 111110. DOI: 10.1016/j.mce.2020.111110. [36] XIE Z, SU W, LIU S, et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation[J]. J Clin Invest, 2015, 125(1): 324-336. DOI: 10.1172/JCI76881. [37] KOWLURU A. Oxidative stress in cytokine-induced dysfunction of the pancreatic beta cell: known knowns and known unknowns[J]. Metabolites, 2020, 10(12): 480. DOI: 10.3390/metabo10120480. [38] YE L, XU WH, XUE J, et al. The clock gene Bmal1 regulates pancreatic β-cell apoptosis via oxidative stress signaling pathway[J]. Zhejiang Med J, 2019, 41(24): 2580-2583. DOI: 10.12056/j.issn.100.06-2785.叶绿, 许伟红, 薛静, 等. 生物钟基因Bmal1通过氧化应激信号通路调节胰岛β细胞凋亡的研究[J]. 浙江医学, 2019, 41(24): 2580-2583. DOI: 10.12056/j.issn.100.06-2785. [39] YE L, WU HX, XU WH, et al. Effect of Bmal1 Supression on Pancreatic β-Cell Function[J]. Med Info, 2020, 33(3): 66-68. DOI: 10.3969/j.issn.1006-1959. [40] UDOH US, VALCIN JA, SWAIN TM, et al. Genetic deletion of the circadian clock transcription factor BMAL1 and chronic alcohol consumption differentially alter hepatic glycogen in mice[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 314(3): G431-431G447. DOI: 10.1152/ajpgi.00281.2017. [41] HARFMANN BD, SCHRODER EA, KACHMAN MT, et al. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis[J]. Skelet Muscle, 2016, 6: 12. DOI: 10.1186/s13395-016-0082-x. [42] PASCHOS GK, IBRAHIM S, SONG WL, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl[J]. Nat Med, 2012, 18(12): 1768-1777. DOI: 10.1038/nm.2979. [43] QIAO B, ZHOU Y, MA WJ, et al. Intestinal microflora imbalance in non-alcoholic fatty liver disease[J/CD]. Chin J Liver Dis (Electronic Edition), 2020, 12(4): 29-33. DOI:10.3969/j.issn.1674-7380.2020.04.005.乔兵, 周永, 马文洁, 等. 肠道菌群失调在非酒精性脂肪性肝病中研究进展[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(4): 29-33. DOI:10.3969/j.issn.1674-7380.2020.04.005. [44] SNIJDER J, AXMANN IM. The kai-protein clock-keeping track of cyanobacteria's daily life[J]. Subcell Biochem, 2019, 93: 359-391. DOI: 10.1007/978-3-030-28151-9_12. [45] THAISS CA, ZEEVI D, LEVY M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis[J]. Cell, 2014, 159(3): 514-529. DOI: 10.1016/j.cell.2014.09.048. [46] LIANG X, BUSHMAN FD, FITZGERALD GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock[J]. Proc Natl Acad Sci U S A, 2015, 112(33): 10479-10484. DOI: 10.1073/pnas.1501305112. [47] KYOKO OO, HIROSHI K, KAYOKO I, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: Implications in intestinal permeability and susceptibility to colitis[J]. PLoS One, 2014, 9(5): e98016. DOI: 10.1371/journal.pone.0098016. [48] HU D, XIE Z, YE Y, et al. The beneficial effects of intermittent fasting: An update on mechanism, and the role of circadian rhythm and gut microbiota[J]. Hepatobiliary Surg Nutr, 2020, 9(5): 597-602. DOI: 10.21037/hbsn-20-317. [49] FÖRSTERMANN U, XIA N, LI H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis[J]. Circ Res, 2017, 120(4): 713-735. DOI: 10.1161/CIRCRESAHA.116.309326. [50] RANI V, DEEP G, SINGH RK, et al. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies[J]. Life Sci, 2016, 148: 183-193. DOI: 10.1016/j.lfs.2016.02.002. [51] YANG Z, KIM H, ALI A, et al. Interaction between stress responses and circadian metabolism in metabolic disease[J]. Liver Res, 2017, 1(3): 156-162. DOI: 10.1016/j.livres.2017.11.002. [52] PEI JF, LI XK, LI WQ, et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks[J]. Nat Cell Biol, 2019, 21(12): 1553-1564. DOI: 10.1038/s41556-019-0420-4. [53] NAKAHATA Y, BESSHO Y. The circadian NAD+ metabolism: Impact on chromatin remodeling and aging[J]. Biomed Res Int, 2016, 2016: 3208429. DOI: 10.1155/2016/3208429. [54] SANDBICHLER AM, JANSEN B, PEER BA, et al. Metabolic plasticity enables circadian adaptation to acute hypoxia in zebrafish cells[J]. Cell Physiol Biochem, 2018, 46(3): 1159-1174. DOI: 10.1159/000489058. [55] REY G, VALEKUNJA UK, FEENEY KA, et al. The pentose phosphate pathway regulates the circadian clock[J]. Cell Metab, 2016, 24(3): 462-473. DOI: 10.1016/j.cmet.2016.07.024. [56] SCHWARZ DS, BLOWER MD. The endoplasmic reticulum: Structure, function and response to cellular signaling[J]. Cell Mol Life Sci, 2016, 73(1): 79-94. DOI: 10.1007/s00018-015-2052-6. [57] YANG L, CALAY ES, FAN J, et al. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction[J]. Science, 2015, 349(6247): 500-506. DOI: 10.1126/science.aaa0079. [58] HERREMA H, ZHOU Y, ZHANG D, et al. XBP1s is an anti-lipogenic protein[J]. J Biol Chem, 2016, 291(33): 17394-17404. DOI: 10.1074/jbc.M116.728949. [59] MENG H, GONZALES NM, LONARD DM, et al. XBP1 links the 12-hour clock to NAFLD and regulation of membrane fluidity and lipid homeostasis[J]. Nat Commun, 2020, 11(1): 6215. DOI: 10.1038/s41467-020-20028-z. -



 PDF下载 ( 2354 KB)
PDF下载 ( 2354 KB)

 下载:
下载:


