过表达HBx的肝细胞对肝星状细胞增殖和活化的影响及其机制
DOI: 10.3969/j.issn.1001-5256.2021.07.017
Effect of hepatitis B x gene-overexpressed hepatocytes on the proliferation and activation of hepatic stellate cells and related mechanism
-
摘要:
目的 探讨HBV感染对肝星状细胞(HSC)活化的影响及作用机制。 方法 收集2020年11月—2021年1月慢性乙型肝炎患者血浆30份、乙型肝炎肝硬化患者血浆42份、肝细胞癌患者血浆30份及健康体检者(健康对照组)的血浆18份,ELISA法检测血浆和条件培养液中HBx、TGFβ1、多巴胺β羟化酶(DBH)和羟脯氨酸(Hyp)的含量。采用LO2细胞构建过表达HBx稳转株细胞, LO2细胞分为LO2-HBx组(稳定表达HBx)、阴性对照组(LO2-con)、空白组,分别制备LO2-HBx、LO2-con和LO2细胞(Mock)的条件培养基,孵育人HSC株LX-2,分为LX-2/LO2-HBx、LX-2/LO2-con、LX-2/Mock 3组,采用CCK-8法检测各组细胞增殖变化。采用rhTGFβ1刺激LX-2细胞,另采用TGFβ1受体抑制剂处理LX-2/LO2-HBx组细胞。荧光定量PCR或Western Blot法检测LO2细胞中的HBx及上述LX-2细胞中α-SMA、Col1A1、DBH和TGFβ1的表达。多组间比较采用单因素方差分析,进一步比较方法采用Bonferroni法;2组间比较采用t检验;相关性分析采用Pearson法。 结果 LO2-HBx可稳定表达HBx蛋白,其培养上清中TGFβ1含量升高(F=324.701,P<0.01);共培养LX-2/LO2-HBx组细胞发生明显细胞形态变化,出现细胞收缩,胞突明显伸长,胞内脂滴减少,与LX-2/LO2-con组比较其增殖活力明显增强(P<0.05),且α-SMA和Col1A1的mRNA(F值分别为144.712和76.680,P值均<0.01)及蛋白(F值分别为234.142和528.708,P值均<0.001)表达水平升高;LX-2/LO2-HBx组细胞中TGFβ1 mRNA(F=29.382, P<0.01)及DBH mRNA水平升高(F=42.662, P<0.01)。随着rhTGFβ1刺激浓度的增加,LX-2细胞中α-SMA(F=1 794.031,P<0.01)、Col1A1(F=91.340,P<0.01)及DBH(F=2 501.011,P<0.01)表达增加,在rhTGFβ1 10 ng/ml时达到峰值。在LO2-HBx组条件培养液中加入TGFβ1受体抑制剂后LX-2细胞中DBH和Col1A1的表达较对照组下调(t值分别为3.603、5.798,P值均<0.05)。慢性乙型肝炎、乙型肝炎肝硬化、肝细胞癌患者的血浆TGFβ1(F=51.188,P<0.001)、HBx(F=39.227,P<0.001)、DBH(F=34.431,P<0.001)及Hyp(F=16.211,P<0.001)较健康对照组升高,血浆中HBx与TGFβ1、TGFβ1与DBH、Hyp与DBH的表达量呈正相关,r分别为0.931、0.863、0.765(P值均<0.001)。 结论 HBx蛋白可促进LO2细胞分泌TGFβ1,诱导LX-2的增殖和活化,促进肝纤维化的发生,并上调LX-2细胞中TGFβ1及DBH的表达;rhTGFβ1刺激可诱导LX-2活化和DBH表达上调。 Abstract:Objective To investigate the effect of hepatitis B virus (HBV) infection on the activation of hepatic stellate cells (HSCs) and its mechanism of action. Methods A total of 30 plasma samples of chronic hepatitis B patients, 42 plasma samples of hepatitis B cirrhosis patients, 30 plasma samples of hepatocellular carcinoma patients, and 18 plasma samples of the individuals undergoing physical examination were collected from November 2020 to January 2021, and ELISA was used to measure the content of hepatitis B X protein (HBx), transforming growth factor-β1 (TGFβ1), dopamine beta-hydroxylase (DBH), and hydroxyproline (HYP) in plasma and conditioned medium. LO2 cells were used to establish a cell line with stable overexpression of HBx (LO2-HBx) and negative control cells (LO2-con), and a conditioned medium was prepared for LO2-HBx, LO2-Con, and LO2 cells (Mock), respectively; human HSC cell line LX-2 was incubated and divided into LX-2/LO2-HBx, LX-2/LO2-con, and LX-2/Mock groups, and CCK-8 assay was used to measure the change in cell proliferation. LX-2 cells were stimulated by rhTGFβ1, and the cells in the LX-2/LO2-HBx group were treated with a TGFβ1 receptor inhibitor. Quantitative real-time PCR and Western blot were used to measure the expression of HBx in LO2 cells and the expression of alpha-smooth muscle actin (α-SMA), collagen type Ⅰ alpha 1 (Col1A1), DBH, and TGFβ1 in the above LX-2 cells. An analysis of variance was used for comparison between multiple groups, and the Bonferroni method was used for further comparison; the t-test was used for comparison between two groups; the Pearson method was used for correlation analysis. Results LO2-HBx stably expressed HBx protein and showed an increase in the content of TGFβ1 in supernatant (F=324.701, P < 0.01). The co-cultured LX-2/LO2-HBx group had a significant change in cell morphology, with the presence of cell shrinkage, extended cytoplasmic process, and reduced lipid droplets, and compared with the LX-2/LO2-con group, the LX-2/LO2-HBx group had significant increases in proliferative activity (P < 0.05) and the mRNA and protein expression levels of α-SMA and Col1A1 (mRNA: F=144.712 and 76.680, both P < 0.01; protein: F=234.142 and 528.708, both P < 0.001). The LX-2/LO2-HBx group had significant increases in the content of TGFβ1 (F=29.382, P < 0.01) and DBH (F=42.662, P < 0.01). With the increase in the stimulating concentration of rhTGFβ1, there were significant increases in the expression of α-SMA (F=1 794.031, P < 0.01), Col1A1 (F=91.340, P < 0.01), and DBH (F=2 501.011, P < 0.01), which reached the peak values at the rhTGFβ1 concentration of 10 ng/ml, and after a TGFβ1 receptor inhibitor was added to the conditioned medium, the LO2-HBx group had significant reductions in the expression of DBH and Col1A1 compared with the control group (t=3.603 and 5.798, both P < 0.05). Compared with the healthy control group, the chronic hepatitis B, liver cirrhosis, and hepatocellular carcinoma groups had significant increases in the plasma levels of TGFβ1 (F=51.188, P < 0.001), HBx (F=39.227, P < 0.001), DBH (F=34.431, P < 0.001), and HYP (F=16.211, P < 0.001), and a positive correlation was observed between plasma HBx and TGFβ1, between TGFβ1 and DBH, and between HYP and DBH (r= 0.931, 0.863, and 0.765, all P < 0.001). Conclusion HBx protein can promote the secretion of TGFβ1 in LO2 cells, induce the proliferation and activation of LX-2 cells, promote the development of liver fibrosis, and upregulate the expression of TGFβ1 and DBH in LX-2 cells, and rhTGFβ1 stimulation can induce the activation of LX-2 cells and the upregulation of DBH expression. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Hepatic Stellate Cells /
- HBV-X Protein
-
HBV的持续感染是导致肝炎肝硬化和肝细胞癌发生最主要的因素之一[1]。慢性HBV感染者比未感染者发生肝癌的风险高25~37倍[2]。部分HBV感染者将经历漫长的肝细胞损伤,修复,再损伤,进而发生肝纤维化,如不及时干预,最终可能在肝硬化的基础上进展为肝细胞癌[3]。肝星状细胞(HSC)的持续激活是肝纤维化发生发展过程中的关键环节,HSC是细胞外基质的主要来源,各种致纤维化因素均把HSC作为最终靶细胞[4]。正常情况下HSC处于静止状态,当肝脏受到炎症或机械刺激等损伤时,HSC被激活。肝细胞是肝脏的实质细胞,占肝脏细胞数量的80%,导致肝细胞损伤的疾病通常会引起肝实质损伤以及肝脏微环境的改变,直接或间接地促进HSC活化[5]。
本研究拟通过应用稳定表达HBx蛋白的LO2细胞系的培养上清液作为条件培养基干预人HSC系LX-2来模拟HBV感染肝细胞的状态,探索HBV感染的肝细胞对HSC活化和增殖的影响及可能的机制。
1. 材料与方法
1.1 材料
1.1.1 临床样本
收集2020年11月—2021年1月在南方医科大学深圳医院诊断为慢性乙型肝炎患者血浆30份、乙型肝炎肝硬化患者血浆42份、肝细胞癌患者血浆30份及同期在本院体检的健康人群血浆18份。
1.1.2 细胞和试剂
人HSC株LX-2、人肝细胞株LO2(中科院上海细胞库);HBx过表达和对照慢病毒(广州莱德尔生物);培养基及胎牛血清(美国Gibco);鼠抗人α-平滑肌肌动蛋白(α-SMA)、鼠抗人α1-Ⅰ型胶原(Col1A1)、鼠抗人GAPDH抗体均为美国Proteintech公司生产;HBV X蛋白抗体(英国Abcam);CCK-8检测试剂(日本同仁);逆转录(美国ThermoFisher);荧光定量PCR试剂(天根生物);TGFβ1、HBxAg、多巴胺β羟化酶(DBH)和Hyp酶联免疫吸附试验(ELISA)试剂盒(上海酶联生物);细胞因子TGFβ1(美国Peprotech),TGFβ1受体抑制剂SB-431542(美国Sigma)。
1.2 方法
1.2.1 细胞培养
LO2细胞采用RP-MI1640培养基,LX-2采用DMEM培养基, 分别添加10%胎牛血清、0.1 mg/ml链霉素、100 U/ml青霉素,培养于5%CO2、37 ℃恒温培养箱。实验取用对数生长期细胞。
1.2.2 稳转株构建
将贴壁细胞以1×105/孔铺到24孔板中。在细胞培养贴壁18~24 h后,吸去细胞原有培养基,加入1 ml新鲜培养基(含40 μl HitrasG P)。以MOI=50加入病毒。摇匀后,置于37 ℃培养箱中感染12~16 h。第2天(12~16 h后)用新鲜培养基替换含有病毒的培养基。继续培养48 h后观察荧光表达。在完全培养基中加入终浓度为1 μg/ml的puro,替换原来的培养基[LO2细胞分为LO2-HBx组(稳定表达HBx)、阴性对照组(LO2-con)、空白组(正常培养的不转病毒的LO2细胞,Mock)]。每天换含puro的完全培养液1次,直到正常培养组细胞被puro杀光。将获得的稳转株扩大传代培养,并冻存。
1.2.3 制备条件培养基培养LX-2细胞
分别将LO2-HBx、LO2-con和未转染肝细胞(Mock)的3组细胞株以1×105密度接种于6孔板,待细胞培养至80%~90%融合度时更换为不含胎牛血清的RPMI 1640培养液,48 h后吸取培养基,离心后取上清制备成条件培养基。
1.2.4 CCK-8法检测各组细胞在共培养0、24、48 h后的增殖变化
当细胞密度达到90%左右时,弃培养基,消化细胞并计数,以每孔5000个细胞接种到96孔板中,每组6个复孔,将培养板放在培养箱中培养过夜(37 ℃,5%CO2)。细胞贴壁6 h后,记为0 h,并进行CCK-8检测;以此时开始计算,此后24、48 h再进行CCK-8检测。分别在0、24、48 h向培养孔分别加入10 μl CCK-8溶液,置于培养箱中孵育1.5 h后用450 nm波长测定各孔吸光度值。
1.2.5 qRT-PCR检测基因表达
以GAPDH作为内参,检测HBx、α-SMA、COL1A1、TGFβ1、DBH mRNA的表达;采用Trizol法提取细胞总RNA,按照反转录试剂盒的说明书进行RNA逆转录,合成cDNA。按照qRT-PCR试剂盒说明书进行进行PCR反应。
1.2.6 Western Blot检测细胞蛋白表达量
Western Blot检测稳转细胞株中HBx蛋白的表达量及条件培养基孵育的LX-2中α-SMA和Col1A1蛋白的表达。采用含50 mmol/L Tris - HCl (pH=7.4), 150 mmol/L NaCl, 0.5%脱氧胆酸钠,0.1% 十二烷基硫酸钠和蛋白酶抑制剂及1 mmol/L RIPA裂解液分离蛋白质。BCA蛋白浓度测定试剂盒检测总蛋白量,调整蛋白浓度为1 μg/μl,上样体积为20 μl。
1.2.7 ELISA检测条件培养液及血浆中TGFβ1、HBxAg、DBH和Hyp的含量
在酶标包被板上设置标准品孔和样本孔,标准品孔各加不同浓度的标准品50 μl。待测样品孔中先加样品稀释液25 μl,然后再加待测样品25 μl,按照ELISA试剂盒说明书操作。以空白孔调零,450 nm波长测量各孔的吸光值。
1.3 伦理学审查
本研究经南方医科大学深圳医院医院医学伦理委员会批准,批号:NYSZYYEC20190005。
1.4 统计学方法
采用SPSS 19.0软件进行统计分析,计量资料以x±s表示,3组间比较采用单因素方差分析, 进一步两两比较采用Bonferroni法;2组间比较采用t检验。采用GraphPad Prism 8.0.1分析相关数据并做统计图,相关性分析采用Pearson法。P<0.05为差异有统计学意义。
2. 结果
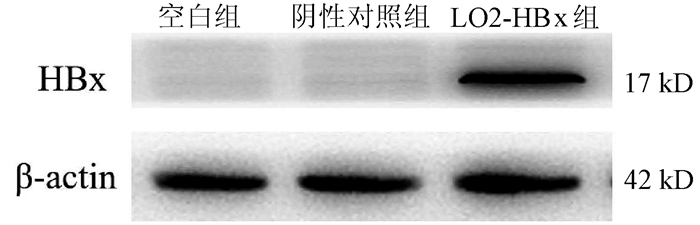
2.1 3组LO2肝细胞中HBx蛋白的表达
成功构建稳定表达HBx基因的LO2稳转株细胞(LO2-HBx),可见除LO2-HBx组细胞外,阴性对照组和空白组细胞中不表达HBx蛋白(图 1)。
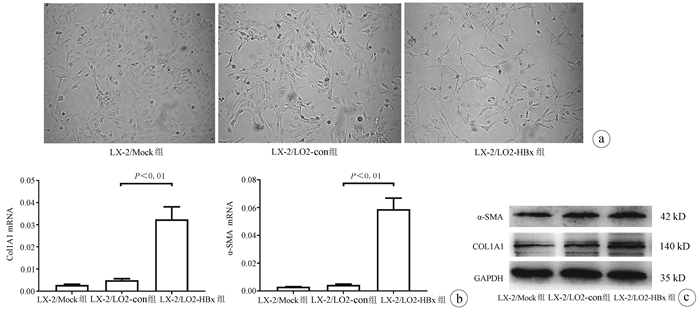
2.2 3组条件培养基培养LX-2细胞对其活化的影响
观察LX-2/LO2-HBx、LX-2/LO2-con、LX-2/Mock 3组共培养体系的LX-2细胞形态,在条件培养48 h后形态出现明显分化,其中LX-2/LO2-HBx组LX-2细胞发生明显细胞形态变化,出现细胞收缩,胞突明显伸长,胞内脂滴减少(图 2a),其α-SMA和Col1A1的mRNA(F值分别为144.712和76.680,P值均<0.01)及蛋白(F值分别为234.142和528.708,P值均<0.001)表达水平均升高(图 2b、c)。
2.3 3组条件培养基培养LX-2细胞对其增殖的影响
LX-2/LO2-HBx组LX-2细胞在条件培养48 h后增殖活力高于LX-2/LO2-con组LX-2细胞(P<0.05)(图 3)。
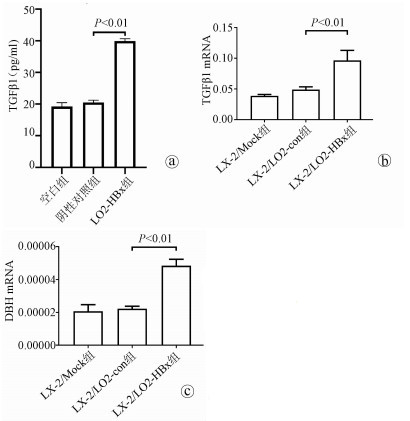
2.4 检测条件培养基中TGFβ1的含量及条件培养后LX-2细胞中TGFβ1与DBH mRNA表达变化
TGFβ1在LO2-HBx组细胞条件培养基中含量升高(F=324.701, P<0.01)(图 4a); 在培养LX-2细胞48 h后提取3组细胞RNA,检测细胞中TGFβ1及DBH mRNA表达量, LX-2/LO2-HBx组LX-2细胞中TGFβ1(F=29.382, P<0.01)(图 4b)及DBH(F=42.662, P<0.01)mRNA表达量均升高(图 4c)。
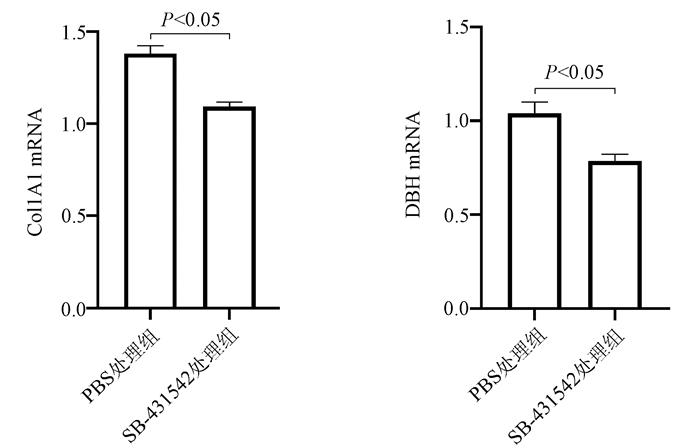
2.5 TGFβ1受体抑制剂处理LX-2/L02-HBx组细胞后DBH的变化
将L02-HBx条件培养基分为2组,分别为10 μmol/L SB-431542处理组(LX-2/LO2-HBx/SB-431542)和PBS处理组(LX-2/LO2-HBx),培养LX-2细胞48 h后提取2组细胞RNA,检测2组细胞中Col1A1及DBH mRNA的表达量。结果发现,加入TGFβ1受体抑制剂的LX-2/LO2-HBx/SB-431542组LX-2细胞中Col1A1(t=5.798, P<0.01)及DBH (t=3.603, P<0.05)mRNA表达量均下降(图 5)。
2.6 重组人TGFβ1(rhTGFβ1)刺激LX-2细胞检测DBH表达变化
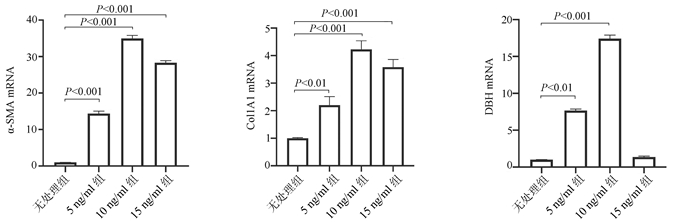
将LX-2细胞分为4组进行处理,分别为: (1)无处理组;(2)加入5 ng/ml rhTGFβ1(5 ng/ml组);(3) 加入10 ng/ml rhTGFβ1(10 ng/ml组);(4)加入15 ng/ml rhTGFβ1(15 ng/ml组);培养48 h后提取4组细胞RNA,检测细胞中α-SMA、Col1A1及DBH mRNA表达水平。结果发现,随着rhTGFβ1刺激浓度的增加,α-SMA(F=1 794.031,P<0.01)、Col1A1(F=91.340,P<0.01) 及DBH(F=2 501.011,P<0.01)表达增加,在rhTGFβ1 10 ng/ml时达到峰值,当rhTGFβ1浓度达到15 ng/ml时,α-SMA、Col1A1及DBH的表达开始由峰值下降(图 6)。
2.7 临床样本中HBx、TGFβ1、Hyp及DBH含量检测
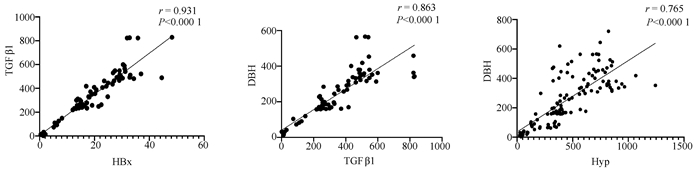
慢性乙型肝炎、乙型肝炎肝硬化、肝细胞癌患者血浆中的HBx、TGFβ1、Hyp及DBH含量均高于健康对照组(P值均<0.001)(表 1);血浆中HBx与TGFβ1之间、TGFβ1与DBH之间、Hyp与DBH之间的表达量呈正相关关系,相关系数r分别为0.931、0.863、0.765(P值均<0.001)(图 7)。
表 1 ELISA检测4组临床样本中HBx、TGFβ1、Hyp及DBH含量组别 例数 HBx(ng/ml) TGFβ1(pg/ml) Hyp(μmol/L) DBH(ng/ml) 健康对照组 18 0.86±0.37 16.01±6.32 201.87±95.64 50.87±20.83 慢性乙型肝炎组 30 26.41±9.161) 471.00±176.011) 591.53±216.401) 365.44±130.621) 乙型肝炎肝硬化组 42 17.87±7.151)2) 500.94±157.621) 652.83±236.171) 378.66±127.131) 肝细胞癌组 30 23.34±9.491) 513.09±163.841) 560.29±195.701) 349.00±138.531) F值 39.227 51.188 16.211 34.431 P值 <0.001 <0.001 <0.001 <0.001 注: 与对照组比较,1)P<0.01, 与慢性乙型肝炎组比较,2)P<0.05。 3. 讨论
微小的肝癌病灶难被发现,且肝脏血供丰富,易发生血行转移,许多患者确诊时已发生转移,失去手术的机会[6]。因此,在肝细胞发生癌变前,找到有效的诊断和治疗靶点,阻断甚至逆转肝炎-肝硬化的进程,对于肝细胞癌的防治十分重要。
HBx蛋白是HBV基因中最小也是最常整合在宿主基因组中的X基因编码的蛋白,可调节细胞基因转录、细胞增殖凋亡及信号传递等,与病毒复制及肝癌发生发展密切相关。细胞及其微环境之间的相互作用可维持组织稳态并对疾病发生发展有着重要影响。本研究通过稳定表达HBx蛋白的LO2细胞系来模拟HBV感染肝细胞的状态,探究HBV感染后细胞微环境变化对HSC的影响。发现稳定表达HBx蛋白的LO2细胞系的培养上清液中TGFβ1含量增加,其制备成的条件培养基可促进HSC LX-2的活化和增殖,并上调HSC LX-2细胞中TGFβ1和DBH的表达。已有许多研究[7]证实TGFβ1是强效促纤维化因子,在肝纤维化中具有重要作用,是HSC活化的重要促进因子。本研究将rhTGFβ1设置不同浓度梯度刺激LX-2细胞,发现随着rhTGFβ1浓度的增加,LX-2的活化标志物α-SMA、Col1A1均表达上调。据此推测HBx可能通过上调TGFβ1促进LX-2细胞的增殖活化。
肝脏的主要生理功能如消化、免疫、代谢、再生等均与神经系统的调控密切相关,交感神经系统在各种肝脏疾病的发生发展中起着重要作用[8],例如交感神经可维持肝细胞癌的炎症微环境,从而促进肝细胞癌的发生[9];交感神经系统通过调节HSC和肝上皮祖细胞的表型来调节肝脏修复;自主神经系统能调节肝脏再生和凋亡[10-11]。DBH可催化多巴胺形成去甲肾上腺素,去甲肾上腺素是交感神经系统的重要递质。美国科学家Oben等[12]研究报道指出,肝纤维化中最重要的一类细胞——HSC, 可以合成和释放去甲肾上腺素,HSC表达多种亚型的肾上腺素受体,能接受交感神经和副交感神经纤维的支配,他们发现Dbh基因缺陷小鼠来源的HSC细胞不能合成去甲肾上腺素,在培养基中增殖缓慢,加入去甲肾上腺素后,增殖能力恢复,并且Dbh基因缺陷小鼠在肝损伤后的纤维化发生也受到阻碍。Coll等[13]对实施门静脉结扎的大鼠模型和肝硬化大鼠模型的肠系膜动脉样本中检测到50个与神经传递特别是肾上腺素能相关的差异表达基因,其中DBH、Th和Snap25下调最明显,这些基因的表达下调可能与门静脉高压的高动力循环状态下内脏和周围血管舒张有关。现有的相关文献提示DBH可能在肝硬化发展过程中有重要作用。但目前为止,DBH的研究大部分集中在神经系统疾病,如帕金森病、精神分裂症等,其在肝脏疾病中的研究十分罕见。本研究首次发现TGFβ1可诱导LX-2细胞中DBH的表达,DBH的含量随着rhTGFβ1浓度的升高而增加,当高浓度的rhTGFβ1(15 ng/ml)处理时,DBH表达较峰值明显下调,LX-2的活化标志物α-SMA、Col1A1表达也从峰值开始下降。在条件培养液中加入TGFβ1受体抑制剂后DBH和Col1A1的表达下调。据此结果推测在一定浓度范围内,TGFβ1可诱导DBH的表达,促进HSC的增殖活化。但TGFβ1具体如何作用使DBH表达升高,DBH又是如何参与了HSC的活化,这些问题还需深入的功能和机制研究来明确。
为了进一步探究HBx、TGFβ1、DBH在慢性乙型肝炎发展不同阶段的表达情况及相关性,本研究分别检测了健康体检者、慢性乙型肝炎、乙型肝炎肝硬化、肝细胞癌患者血浆中HBx、TGFβ1、DBH和Hyp的表达量。Hyp是一种非必需氨基酸,是胶原蛋白中含量最多的氨基酸,在胶原合成中起着至关重要的作用[14]。胶原蛋白是构成结缔组织中胶原纤维的主要成分,通过血浆Hyp的测定,可了解体内胶原蛋白分解代谢情况,因此常被用于评估HSC的激活和肝硬化疾病状态[15-16]。本研究发现HBx、TGFβ1、DBH、Hyp在慢性乙型肝炎、乙型肝炎肝硬化及肝细胞癌患者血浆中均明显升高,且HBx与TGFβ1、TGFβ1与DBH之间相关性强,从临床水平验证了细胞实验得到的HBx可诱导TGFβ1分泌,TGFβ1又可促进DBH表达的实验结果。本研究首次在临床样本中发现慢性乙型肝炎、乙型肝炎肝硬化及肝细胞癌患者血浆中DBH的含量明显升高,且DBH与血浆中TGFβ1和Hyp的表达量呈正相关关系,提示DBH可能与慢性乙型肝炎-肝硬化发展过程有关,为乙型肝炎相关疾病的研究提供了新线索。在将来的研究中需扩大样本量检测,并将DBH含量与其他肝功能及肝纤维化指标联合分析,同时进行动物实验并深入机制研究,将更有利于阐明DBH在慢性乙型肝炎-肝硬化-肝癌发展过程中的作用及机制。
-
表 1 ELISA检测4组临床样本中HBx、TGFβ1、Hyp及DBH含量
组别 例数 HBx(ng/ml) TGFβ1(pg/ml) Hyp(μmol/L) DBH(ng/ml) 健康对照组 18 0.86±0.37 16.01±6.32 201.87±95.64 50.87±20.83 慢性乙型肝炎组 30 26.41±9.161) 471.00±176.011) 591.53±216.401) 365.44±130.621) 乙型肝炎肝硬化组 42 17.87±7.151)2) 500.94±157.621) 652.83±236.171) 378.66±127.131) 肝细胞癌组 30 23.34±9.491) 513.09±163.841) 560.29±195.701) 349.00±138.531) F值 39.227 51.188 16.211 34.431 P值 <0.001 <0.001 <0.001 <0.001 注: 与对照组比较,1)P<0.01, 与慢性乙型肝炎组比较,2)P<0.05。 -
[1] VITTAL A, GHANY MG. WHO guidelines for prevention, care and treatment of individuals infected with HBV: A US perspective[J]. Clin Liver Dis, 2019, 23(3): 417-432. DOI: 10.1016/j.cld.2019.04.008. [2] WANG M, XI D, NING Q. Virus-induced hepatocellular carcinoma with special emphasis on HBV[J]. Hepatol Int, 2017, 11(2): 171-180. DOI: 10.1007/s12072-016-9779-5. [3] BAGLIERI J, BRENNER DA, KISSELEVA T. The Role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma[J]. Int J Mol Sci, 2019, 20(7). DOI: 10.3390/ijms20071723. [4] ZHU J, LUO Z, PAN Y, et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes[J]. J Cell Physiol, 2019, 234(6): 9698-9710. DOI: 10.1002/jcp.27656. [5] XU Y, ZHANG DQ, CHEN JM, et al. Effect of various cells on the activation of hepatic stellate cells in liver microenvironment[J]. J Clin Hepatol, 2019, 35(2): 424-430. DOI: 10.3969/j.issn.1001-5256.2019.02.042.徐莹, 张定棋, 陈佳美, 等. 肝脏微环境中各种细胞对肝星状细胞活化的影响[J]. 临床肝胆病杂志, 2019, 35(2): 424-430. DOI: 10.3969/j.issn.1001-5256.2019.02.042. [6] EL-SERAG HB, MARRERO JA, RUDOLPH L, et al. Diagnosis and treatment of hepatocellular carcinoma[J]. Gastroenterology, 2008, 134(6): 1752-1763. DOI: 10.1053/j.gastro.2008.02.090. [7] REN CZ, HAO LS. Signal transduction involved in activation of hepatic stellate cells[J]. J Clin Hepatol, 2015, 31(3): 452-456. DOI: 10.3969/j.issn.1001-5256.2015.03.034.任昌镇, 郝礼森. 肝星状细胞活化过程中的信号转导[J]. 临床肝胆病志, 2015, 31(3): 452-456. DOI: 10.3969/j.issn.1001-5256.2015.03.034. [8] WEN X, HUAN H, WANG X, et al. Sympathetic neurotransmitters promote the process of recellularization in decellularized liver matrix via activating the IL-6/Stat3 pathway[J]. Biomed Mater, 2016, 11(6): 065007. DOI: 10.1088/1748-6041/11/6/065007. [9] HUAN HB, WEN XD, CHEN XJ, et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells[J]. Brain Behav Immun, 2017, 59: 118-134. DOI: 10.1016/j.bbi.2016.08.016. [10] KAMIMURA K, INOUE R, NAGOYA T, et al. Autonomic nervous system network and liver regeneration[J]. World J Gastroenterol, 2018, 24(15): 1616-1621. DOI: 10.3748/wjg.v24.i15.1616. [11] WANG L, ZHU L, WU K, et al. Mitochondrial general control of amino acid synthesis 5 like 1 regulates glutaminolysis, mammalian target of rapamycin complex 1 activity, and murine liver regeneration[J]. Hepatology, 2020, 71(2): 643-657. DOI: 10.1002/hep.30876. [12] OBEN JA, ROSKAMS T, YANG S, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters[J]. Gut, 2004, 53(3): 438-445. DOI: 10.1136/gut.2003.026658. [13] COLL M, GENESCÀ J, RAURELL I, et al. Down-regulation of genes related to the adrenergic system may contribute to splanchnic vasodilation in rat portal hypertension[J]. J Hepatol, 2008, 49(1): 43-51. DOI: 10.1016/j.jhep.2008.03.015. [14] LI P, WU G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth[J]. Amino Acids, 2018, 50(1): 29-38. DOI: 10.1007/s00726-017-2490-6. [15] SRIVASTAVA AK, KHARE P, NAGAR HK, et al. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases[J]. Curr Protein Pept Sci, 2016, 17(6): 596-602. DOI: 10.2174/1389203717666151201192247. [16] SHIN SK, KIM KO, KIM SH, et al. Exogenous 8-hydroxydeoxyguanosine ameliorates liver fibrosis through the inhibition of Rac1-NADPH oxidase signaling[J]. J Gastroenterol Hepatol, 2020, 35(6): 1078-1087. DOI: 10.1111/jgh.14979. 期刊类型引用(3)
1. 柯畅,刘艳菊,涂济源,张方蕾,高建龙,周仲实. 基于代谢组学研究肝复乐胶囊抑制LX-2细胞激活机制. 中成药. 2024(02): 672-678 .  百度学术
百度学术2. 王磊,金香淑,董慧君,欧国敏,赖鑫源,庄辉,李彤,向宽辉. 基于COL1A1启动子和增强型绿色荧光蛋白基因建立人肝星状细胞活化的细胞模型. 北京大学学报(医学版). 2023(05): 876-885 .  百度学术
百度学术3. 党中峰,张永成,赵凤菊,马亚兵,党雅梅. 乙肝病毒X蛋白可通过调控CXC趋化因子受体6表达促进肝癌细胞的侵袭和转移. 中华实验外科杂志. 2023(10): 1962-1965 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3213 KB)
PDF下载 ( 3213 KB)


 下载:
下载:






 下载:
下载:

 百度学术
百度学术








