癌旁组织二酯酰甘油激酶γ表达水平对肝细胞癌患者术后生存的影响
DOI: 10.3969/j.issn.1001-5256.2021.05.023
Effect of the expression level of diacylglycerol kinase gamma in paracancerous tissue on postoperative survival in patients with hepatocellular carcinoma
-
摘要:
目的 探究肝癌患者癌旁组织中二酯酰甘油激酶γ(DGKγ)的表达水平对术后生存的影响及临床价值。 方法 收集2008年12月—2012年8月郑州大学附属肿瘤医院收治的78例行手术切除的肝癌患者资料。实时荧光定量PCR检测癌旁组织中DGKγmRNA的表达水平,将78例患者分为低表达组(DGKγ<0.0862,简称LEP组)和高表达组(DGKγ≥0.0862,简称HEP组),比较2组间的基本资料特征。计量资料2组间比较使用t检验与Mann-Whitney U检验;计数资料2组间比较使用χ2检验。单因素和多因素Cox回归分析患者生存预后的独立影响因素;Kaplan-Meier法分析所有患者和巴塞罗那(BCLC)分期各亚组中LEP组和HEP组的生存情况。 结果 多因素Cox分析显示:癌旁DGKγ表达水平(HR=1.913,95%CI:1.111~3.296,P=0.019)、HBsAg(HR=2.645,95%CI:1.264~5.537,P=0.010)、Alb(HR=0.952,95%CI:0.916~0.990,P=0.013)、BCLC分期(HR=1.702,95%CI:1.267~2.286,P<0.001)、肿瘤大小(HR=1.083,95%CI:1.019~1.152,P=0.011)是肝癌患者术后长期生存的独立影响因素。78例患者中,LEP组的中位生存时间为45.0个月,显著高于HEP组的22.9个月(P=0.0025)。分层分析显示,BCLC A期中,LEP组的远期生存情况显著的优于HEP组(P=0.0345);B和C期中,LEP组和HEP组中位生存时间分别为16.5个月和10.8个月,2组间的近期和远期生存差异无统计学意义(P>0.05)。 结论 癌旁组织中DGKγ的表达水平可能是一种新的能够预测和评估肝癌患者术后长期生存风险的指标,具有一定的临床应用价值。 Abstract:Objective To investigate the effect of the expression level of diacylglycerol kinase gamma (DGKγ) in paracancerous tissue on the postoperative prognosis of patients with hepatocellular carcinoma (HCC) and its clinical value. Methods Related clinical data were collected from 78 HCC patients who were admitted and underwent surgical resection from December 2008 to August 2012 in the Affiliated Cancer Hospital of Zhengzhou University. Quantitative real-time PCR was used to measure the mRNA expression level of DGKγ in paracancerous tissue, and then the 78 patients were divided into low expression group (DGKγ < 0.086 2, LEP group) and high expression group (DGKγ ≥0.086 2, HEP group). Basic data and clinical features were compared between the two groups. The t-test and the Mann-Whitney U test were used for comparison of continuous data, and the chi-square test and the corrected chi-square test were used for comparison of categorical data. Univariate and multivariate Cox regression analyses were used to investigate independent influencing factors for survival and prognosis, and the Kaplan-Meier method was used to analyze the overall survival trends of all patients and the LEP and HEP groups in each subgroup of Barcelona Clinic Liver Cancer (BCLC) stages. Results The multivariate Cox regression analysis showed that the expression level of DGKγ (HR=1.913, 95%CI: 1.111-3.296, P=0.019), HBsAg (HR=2.645, 95%CI: 1.264- 5.537, P=0.010), Alb (HR=0.952, 95%CI: 0.916-0.990, P=0.013), BCLC stage (HR=1.702, 95%CI: 1.267-2.286, P < 0.001) and tumor size (HR=1.083, 95%CI: 1.019-1.152, P=0.011) were independent influencing factors for long-term survival of HCC patients; the LEP group had a significantly longer median survival time than the HEP group (45.0 months vs 22.9 months, P= 0.002 5). The stratified analysis showed that for BCLC stage A HCC, the LEP group had significantly better long-term survival than the HEP group (P=0.034 5); for BCLC stage B/C HCC, the LEP group had a longer median survival time than the HEP group (16.5 months vs 10.8 months), but there was no significant difference in short- and long-term survival between the two groups (P > 0.05). Conclusion The expression level of DGKγ in paracancerous tissue may be a new index for predicting and evaluating the long-term survival risk of HCC patients after surgery and has certain value in clinical application. -
Key words:
- Liver Neoplasms /
- Diglycerides /
- Prognosis
-
肝细胞癌(HCC,简称肝癌)居我国最常见恶性肿瘤的第四位及肿瘤致死病因的第二位,严重威胁着人民的生命健康[1-3],肝切除术是肝癌的主要治疗方式之一[4],虽然近年来肝癌的外科治疗技术不断发展,但患者的长期预后并不十分理想[5-7]。因此,临床上针对肝癌的多学科综合治疗受到越来越多的重视[5-6]。评估肝癌切除术后患者的预后并为其制订科学合理的综合治疗方案对于改善术后生存至关重要,而目前这一过程的实现仍面临着一些挑战。
二酯酰甘油激酶(DGK)是使二脂酰甘油(DAG)磷酸化产生磷脂酸(PA)的一类激酶;通过对DAG和PA作为第二信使所参与的体内诸多生理信号通路的调节,DGK在许多肿瘤的发生发展中发挥着重要作用[8-10]。DGKγ是DGK家族的Ⅰ型成员之一,目前关于DGKγ在肿瘤中的作用研究比较有限。除了Kai等[11]报道的DGKγ在结直肠癌中发挥抑癌作用外,作者的前期研究[12]发现DGKγ在肝癌的发生发展中扮演着肿瘤抑制因子的角色。此外,该研究还发现相比于肝癌组织中DGKγ的低表达,癌旁组织中DGKγ的表达水平较高;且相比于肝癌组织中DGKγ高表达组的32.87个月的中位生存期,癌/癌旁组织中DGKγ非低表达组的中位生存期则为50.43个月[12]。这些结果提示了癌旁组织中DGKγ的表达水平可能对肝癌患者的预后也存在影响,但目前尚无对此的直接研究报道。本文通过相关的比较和生存分析,首次探究了肝癌患者癌旁组织中DGKγ的表达水平与肝癌术后预后的直接关系,并评估了其作为预后指标的临床价值。
1. 资料与方法
1.1 研究对象
选取2008年12月—2012年8月于郑州大学附属肿瘤医院被诊断为肝癌并行手术切除术的患者82例;经长期随访最终入组的患者为78例,随访率为95.1%。78例患者均为伴有肝硬化的肝癌患者,其中男67例,女11例,平均(54.32±10.74)岁。纳入的患者符合:肝肿瘤手术切除,术后病理证实为HCC;一般情况较好,肝功能Child分级为A级或B级;术前未进行放化疗。排除临床资料不完整、合并有血液及免疫系统疾病及失访的患者。长期随访采用医院就诊和电话相结合的方式,第1年每3个月随访1次,随后每6个月随访1次,最后随访日期为2018年7月1日。
1.2 临床资料的采集
收集研究对象的年龄、性别等临床基本信息;术前实验室检查:HBsAg、AFP、ALT、AST、ALP、GGT、TBil、Alb、球蛋白(Glb)、RBC、PLT、PT、国际标准化比值(INR)、纤维蛋白原(FIB);以及术中输血、肿瘤大小和数目、癌栓、切缘等资料。
1.3 癌旁组织中DGKγ的检测
取手术切除肿瘤后距离肿瘤边缘1~2 cm范围的肝组织50 mg作为癌旁组织样本,-80 ℃冻存保存,在研钵中加入适量的液氮充分研磨,之后收集至10 ml离心管中,加入1 ml TRIzol吹打混匀,室温静置5 min。然后提取RNA,经逆转录后,以C末端结合蛋白1为内参基因,通过实时荧光定量PCR(SYBR Green I Master Mix, Roche)检测癌旁组织中DGKγmRNA的表达水平。
1.4 伦理学审查
本研究方案经由北京大学生物医学伦理委员会审批,批号:IRB00001052-12088,患者均知情同意。
1.5 统计学方法
应用SPSS 24.0统计软件进行数据分析。符合正态分布的计量资料以x±s表示,2组间比较采用t检验;非正态分布的计量资料以M(P25~P75)表示,2组间比较采用Mann-Whitney U检验;计数资料2组间比较使用χ2检验。应用单因素Cox分析找出影响术后总体生存期(OS)的显著因素,然后通过多因素Cox回归分析(向前法)找出术后OS的独立影响因素,结果以HR及其95%CI表示。生存分析使用Kaplan-Meier法及log-rank检验、Breslow检验。P<0.05为差异有统计学意义。
2. 结果
2.1 癌旁组织DGKγ低表达组与高表达组的临床资料比较
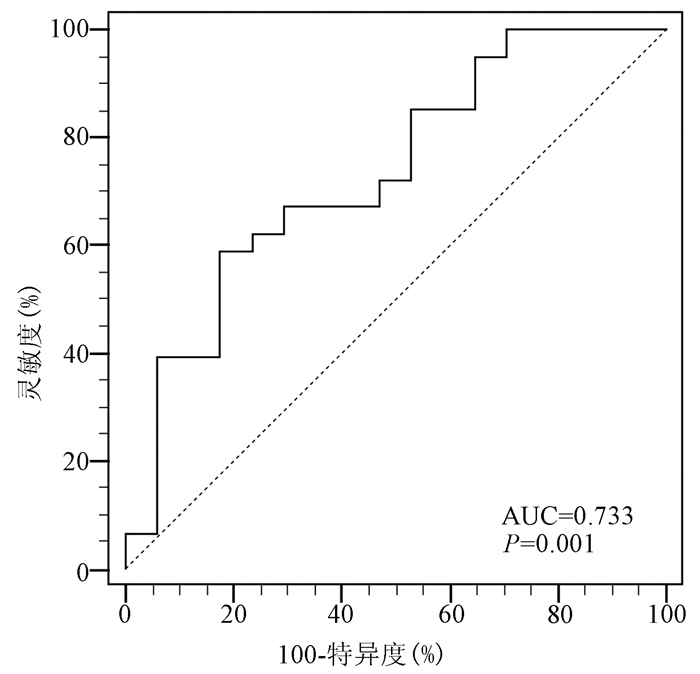
78例HCC患者癌旁DGKγ表达水平分别为0.079 5 (0.007 0~1.697 5)。通过受试者工作特征曲线(ROC曲线)分析78例HCC患者的癌旁DGKγ检测值对患者长期生存状态的预测能力,曲线下面积(AUC)为0.733(P=0.001),确定癌旁DGKγ的最佳截断界值为0.086 2(图 1)。以0.086 2为界,将78例患者分为低表达组(DGKγ<0.086 2,简称LEP组, n=41)和高表达组(DGKγ≥0.086 2,简称HEP组, n=37)。
78例患者的临床资料如表 1所示,18例HBsAg阴性的患者中抗-HBc和抗-HCV阳性的患者分别为4例和3例。比较肝癌患者癌旁组织DGKγ LEP组与HEP组的临床资料,结果显示:LEP组中的PT低于HEP组,差异具有统计学意义(P<0.05);而2组间的年龄、性别、HBsAg(阳性/阴性)、术中输血、BCLC分期、肿瘤大小和数目及其他的实验室指标的差异均无统计学意义(P值均>0.05)(表 1)。
表 1 癌旁组织DGKγ LEP组与HEP组的临床资料比较指标 总患者(n=78) LEP组(n=41) HEP组(n=37) 统计值 P值 年龄(岁) 54.32±10.74 55.17±10.62 53.38±10.94 t=0.734 0.465 性别(男/女) 67/11 33/8 34/3 χ2=1.253 0.263 HBsAg(阳性/阴性) 60/18 28/13 32/5 χ2=2.674 0.102 AFP(ng/μl) 420.2(12.7~1210.0) 237.6(4.3~1039.0) 532.4(21.1~1210.0) Z=-1.621 0.105 ALT(U/L) 38.0(25.0~63.3) 38.0(24.5~55.0) 38.0(25.5~70.5) Z=-0.280 0.779 AST(U/L) 44.0(31.0~66.0) 42.0(32.0~60.0) 49.0(28.5~72.0) Z=-0.756 0.450 ALP(U/L) 113.5(86.8~147.0) 123.0(90.0~153.0) 100.0(85.5~126.0) Z=-1.501 0.133 GGT(U/L) 96.0(56.8~179.0) 117.0(50.5~191.7) 87.0(60.5~150.9) Z=-1.046 0.296 TBil(μmol/L) 15.4(10.9~20.6) 15.5(11.4~20.5) 14.4(9.9~20.7) Z=-0.530 0.596 Alb(g/L) 39.88±6.61 39.71±7.77 40.08±5.11 t=-0.247 0.806 Glb(g/L) 29.56±6.34 30.07±5.79 28.99±6.93 t=0.751 0.455 RBC(×1012/L) 4.31±0.63 4.36±0.59 4.25±0.66 t=0.775 0.441 PLT(×109/L) 151(104~200) 167(113~212) 140(102~186) Z=-1.256 0.209 PT(s) 13.4(12.1~14.4) 13.0(11.7~14.4) 13.7(12.7~14.7) Z=-1.987 0.047 INR 1.15(1.02~1.23) 1.10(0.99~1.25) 1.16(1.08~1.23) Z=-1.392 0.164 FIB(g/L) 3.00±0.85 3.02±0.93 2.97±0.76 t=0.097 0.767 Child-Pugh分级(A/B) 69/9 38/3 31/6 χ2=0.763 0.382 术中输血(是/否) 26/52 12/29 14/23 χ2=0.643 0.423 BCLC分期(A/B/C) 44/10/24 27/4/10 17/6/14 χ2=3.143 0.208 肿瘤大小(cm) 7.0(5.0~11.0) 7.0(4.5~12.0) 8.0(5.0~10.5) Z=-0.431 0.666 肿瘤数目(1个/≥2个) 58/20 33/8 25/12 χ2=1.703 0.192 切缘(是/否) 48/30 26/15 22/15 χ2=0.129 0.720 2.2 肝癌生存预后的单因素及多因素Cox回归分析
单因素Cox分析结果显示HBsAg、AFP、Alb、PLT、术中输血、BCLC分期、肿瘤大小、肿瘤数目、切缘及癌旁组织DGKγ对肝癌患者术后OS的影响差异具有统计学意义(P值均<0.05)(表 2)。共线性诊断分析未发现单因素Cox分析有意义的指标存在共线性;将这些指标纳入多因素Cox回归模型,结果显示癌旁DGKγ(HR=1.913,95%CI:1.111~3.296,P=0.019)、HBsAg(HR=2.645,95%CI:1.264~5.537,P=0.010)、Alb(HR=0.952,95%CI:0.916~0.990,P=0.013)、BCLC分期(HR=1.702,95%CI:1.267~2.286,P<0.001)、肿瘤大小(HR=1.083,95%CI:1.019~ 1.152,P=0.011)是肝癌患者术后OS的独立影响因素;其中癌旁组织DGKγ的高表达是危险因素,HEP组的死亡风险是LEP组的1.913倍。
表 2 所有患者生存预后的单因素分析指标 HR(95%CI) P值 年龄(岁) 0.984(0.960~1.008) 0.186 性别(男/女) 0.775(0.381~1.577) 0.482 HBsAg(阳性/阴性) 2.376(1.198~4.710) 0.013 AFP(ng/μl) 1.001(1.000~1.001) 0.016 ALT(U/L) 1.001(1.000~1.001) 0.293 AST(U/L) 1.001(1.000~1.001) 0.088 ALP(U/L) 1.004(1.000~1.008) 0.080 GGT(U/L) 1.002(1.000~1.004) 0.063 TBil(μmol/L) 0.994(0.966~1.022) 0.660 Alb(g/L) 0.953(0.926~0.981) 0.001 Glb(g/L) 1.007(0.967~1.048) 0.749 RBC(×1012/L) 0.817(0.508~1.314) 0.405 PLT(×109/L) 1.003(1.000~1.006) 0.037 PT(s) 1.059(0.945~1.186) 0.324 INR 2.649(0.825~8.508) 0.102 FIB(g/L) 1.338(0.999~1.791) 0.051 Child-Pugh分级(A/B) 1.517(0.718~3.203) 0.275 术中输血(是/否) 2.430(1.437~4.111) 0.001 BCLC分期(A/B/C) 2.033(1.532~2.699) <0.001 肿瘤大小(cm) 1.088(1.030~1.148) 0.002 肿瘤数目(1个/≥2个) 1.814(1.039~3.167) 0.036 切缘(是/否) 1.859(1.112~3.107) 0.018 癌旁DGKγ (HEP/LEP) 2.188(1.302~3.677) 0.003 2.3 癌旁组织DGKγ LEP组与HEP组的生存曲线比较
基于以上结果,进一步分析比较LEP组与HEP组的生存情况。在78例总患者中,LEP组和HEP组OS近期差异(Breslow检验)与远期差异(log-rank检验) 均具有统计学意义(P值均<0.05,图 2a),中位生存时间分别为45.0和22.9个月;LEP组与HEP组1、3、5年生存率分别为82.9%、56.1%、43.9%和64.9%、24.3%、13.5%。图 2b显示出BCLC A期患者的OS要显著优于BCLC B期和C期的患者,中位生存时间分别为43.9、22.9和11.5个月。随后,分别分析比较了在BCLC A期、BCLC B和C期中LEP组与HEP组的生存差异。结果显示,对于BCLC A期的患者,LEP组和HEP组OS的远期差异具有统计学意义(P<0.05,图 2c),中位生存时间分别为81.9和34.2个月;B期和C期患者中,LEP组和HEP组OS近期与远期差异均无统计学意义(P值均>0.05,图 2d),但前者中位生存时间要高于后者,分别为16.5和10.8个月。
3. 讨论
近年来,全世界及中国的肝癌发病和死亡人数仍然呈现上升趋势[5, 7]。尽管随着临床围手术期管理和诊疗技术的不断进步,对合并有肝硬化的肝癌患者的外科手术干预的短期风险大大降低,但整个肝癌患者人群的长期预后仍不理想[1, 5]。对术后患者进行长期生存风险的评估和预测,并为其制订科学合理的随访复查与及时干预方案以达到改善肝癌患者术后长期生存的目的,是临床医生和研究人员长期努力的方向。既往作者实验室及其他实验室的研究结果表明HBsAg[13]、Alb[14-15]、肿瘤大小[16]和BCLC分期[15, 17]是肝癌患者长期生存的独立影响因素。而本研究中的结果也显示这些指标对肝癌患者术后的长期生存有着一定的预测作用,同时这些风险因素也反映出肝癌患者的长期生存风险是受到了病毒性肝炎、肝功能及肿瘤本身的综合影响作用。
DAG是脂质代谢过程中的中间产物,作为一种中性脂质也是细胞膜的重要构成成分;DAG经DGK磷酸化后可产生PA[18]。在体内许多的生物学进程中,DAG和PA作为关键的第二信使执行着特殊的功能任务,而DGK磷酸化DAG也是信使DAG的主要代谢方式[18]。不同亚型的DGK可以通过终止DAG信号和激活PA介导的通路来调控多种细胞内过程。近年来,关于DGK在肿瘤的增殖、侵袭和转移等生物学进程中的作用受到越来越多的重视。研究较多的是DGKα,目前已知其在乳腺癌[19]、子宫内膜癌[20]、神经胶质母细胞瘤[21]和血液系统肿瘤[22]等恶性肿瘤中主要扮演着促癌基因的功能。这可能与肿瘤微环境能够促进DGKα的表达有关;一方面,DGKα介导生成的PA能够促进肿瘤的生存、迁移和耐药性的产生;另一方面,DGKα所介导的DAG磷酸化代谢能够减弱T淋巴细胞的应答反应并可使T淋巴细胞进入无应答状态[10]。而在胃癌和结直肠癌中,DGKα的表达则是下调的,可能主要发挥了抑癌基因的功能[23-24]。对于肝癌,Takeishi等[25]研究发现表达上调的DGKα依赖于其激酶活性,可通过激活丝裂原活化蛋白激酶(MAPK)通路促进肝癌细胞的增殖和肿瘤进展。
不同于DGKα,作者的前期研究发现DGKγ可通过其激酶活性下调肝癌细胞葡萄糖转运体1的水平而发挥抑癌作用;而相比于肝癌组织中低表达DGKγ的患者,高表达组有着更长的中位生存时间(21.17个月vs 32.87个月, P=0.0092)[12]。然而,本研究的结果发现癌旁组织中的DGKγ表达水平也是肝癌患者术后长期生存的独立影响因素,癌旁组织中DGKγ的水平越低,患者的术后总体生存越好;在BCLC不同分期的亚组分析中也得到了相似的结果。不同于DGKγ在癌组织中发挥抑癌因子的作用,癌旁组织中DGKγ的表达水平对肝癌患者术后生存影响的具体机制可能与DGKγ参与DAG和PA代谢进而长期影响肝脏的代谢功能有关。一方面,癌旁组织DGKγ LEP组中肝功能Child-Pugh B级患者占7.3%,要低于HEP组的16.2%;另一方面前者的PT要显著地低于后者;这些差异提示了癌旁组织中DGKγ可能通过调节肝脏的合成代谢功能来影响肝癌患者的预后。
综上所述,对于肝癌患者,癌旁组织中DGKγ的表达水平是术后长期生存的独立影响因素,可能是一种新的肝癌预后指标;其能够在一定程度上帮助临床医师对术后患者的长期生存风险进行预测和评估,从而更好地帮助临床决策。此外,本研究尚存在着样本量少的局限性,扩大研究的样本量及探索相关的具体作用机制是下一步的研究方向。
-
表 1 癌旁组织DGKγ LEP组与HEP组的临床资料比较
指标 总患者(n=78) LEP组(n=41) HEP组(n=37) 统计值 P值 年龄(岁) 54.32±10.74 55.17±10.62 53.38±10.94 t=0.734 0.465 性别(男/女) 67/11 33/8 34/3 χ2=1.253 0.263 HBsAg(阳性/阴性) 60/18 28/13 32/5 χ2=2.674 0.102 AFP(ng/μl) 420.2(12.7~1210.0) 237.6(4.3~1039.0) 532.4(21.1~1210.0) Z=-1.621 0.105 ALT(U/L) 38.0(25.0~63.3) 38.0(24.5~55.0) 38.0(25.5~70.5) Z=-0.280 0.779 AST(U/L) 44.0(31.0~66.0) 42.0(32.0~60.0) 49.0(28.5~72.0) Z=-0.756 0.450 ALP(U/L) 113.5(86.8~147.0) 123.0(90.0~153.0) 100.0(85.5~126.0) Z=-1.501 0.133 GGT(U/L) 96.0(56.8~179.0) 117.0(50.5~191.7) 87.0(60.5~150.9) Z=-1.046 0.296 TBil(μmol/L) 15.4(10.9~20.6) 15.5(11.4~20.5) 14.4(9.9~20.7) Z=-0.530 0.596 Alb(g/L) 39.88±6.61 39.71±7.77 40.08±5.11 t=-0.247 0.806 Glb(g/L) 29.56±6.34 30.07±5.79 28.99±6.93 t=0.751 0.455 RBC(×1012/L) 4.31±0.63 4.36±0.59 4.25±0.66 t=0.775 0.441 PLT(×109/L) 151(104~200) 167(113~212) 140(102~186) Z=-1.256 0.209 PT(s) 13.4(12.1~14.4) 13.0(11.7~14.4) 13.7(12.7~14.7) Z=-1.987 0.047 INR 1.15(1.02~1.23) 1.10(0.99~1.25) 1.16(1.08~1.23) Z=-1.392 0.164 FIB(g/L) 3.00±0.85 3.02±0.93 2.97±0.76 t=0.097 0.767 Child-Pugh分级(A/B) 69/9 38/3 31/6 χ2=0.763 0.382 术中输血(是/否) 26/52 12/29 14/23 χ2=0.643 0.423 BCLC分期(A/B/C) 44/10/24 27/4/10 17/6/14 χ2=3.143 0.208 肿瘤大小(cm) 7.0(5.0~11.0) 7.0(4.5~12.0) 8.0(5.0~10.5) Z=-0.431 0.666 肿瘤数目(1个/≥2个) 58/20 33/8 25/12 χ2=1.703 0.192 切缘(是/否) 48/30 26/15 22/15 χ2=0.129 0.720 表 2 所有患者生存预后的单因素分析
指标 HR(95%CI) P值 年龄(岁) 0.984(0.960~1.008) 0.186 性别(男/女) 0.775(0.381~1.577) 0.482 HBsAg(阳性/阴性) 2.376(1.198~4.710) 0.013 AFP(ng/μl) 1.001(1.000~1.001) 0.016 ALT(U/L) 1.001(1.000~1.001) 0.293 AST(U/L) 1.001(1.000~1.001) 0.088 ALP(U/L) 1.004(1.000~1.008) 0.080 GGT(U/L) 1.002(1.000~1.004) 0.063 TBil(μmol/L) 0.994(0.966~1.022) 0.660 Alb(g/L) 0.953(0.926~0.981) 0.001 Glb(g/L) 1.007(0.967~1.048) 0.749 RBC(×1012/L) 0.817(0.508~1.314) 0.405 PLT(×109/L) 1.003(1.000~1.006) 0.037 PT(s) 1.059(0.945~1.186) 0.324 INR 2.649(0.825~8.508) 0.102 FIB(g/L) 1.338(0.999~1.791) 0.051 Child-Pugh分级(A/B) 1.517(0.718~3.203) 0.275 术中输血(是/否) 2.430(1.437~4.111) 0.001 BCLC分期(A/B/C) 2.033(1.532~2.699) <0.001 肿瘤大小(cm) 1.088(1.030~1.148) 0.002 肿瘤数目(1个/≥2个) 1.814(1.039~3.167) 0.036 切缘(是/否) 1.859(1.112~3.107) 0.018 癌旁DGKγ (HEP/LEP) 2.188(1.302~3.677) 0.003 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] TORRE LA, BRAY F, SIEGEL RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. DOI: 10.3322/caac.21262. [3] ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. [4] LIU AX, WANG HQ, BO WT, et al. Clinical efficacy and prognostic factors analysis of hepatectomy for hepatocellular carcinoma[J]. Chin J Dig Surg, 2019, 18(4): 368-374. DOI: 10.3760/cma.j.issn.1673-9752.2019.04.012.刘爱祥, 王海清, 薄文滔, 等. 肝细胞癌肝切除术的临床疗效及预后因素分析[J]. 中华消化外科杂志, 2019, 18(4): 368-374. DOI: 10.3760/cma.j.issn.1673-9752.2019.04.012. [5] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [6] Bureau of Medical Administration, National Health Commission of The People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [7] QIAN XJ, QU CF, LU FM. The tumor markers of hepatocellular carcinoma play an indispensable role in ultrasound screening and monitoring early hepatocellular carcinoma[J]. Liver, 2019, 24(8): 851-853. DOI: 10.3969/j.issn.1008-1704.2019.08.003.钱相君, 曲春枫, 鲁凤民. 肝癌肿瘤标记物在超声筛查监测早期肝细胞癌中的作用不可或缺[J]. 肝脏, 2019, 24(8): 851-853. DOI: 10.3969/j.issn.1008-1704.2019.08.003. [8] HUANG C, FRETER C. Lipid metabolism, apoptosis and cancer therapy[J]. Int J Mol Sci, 2015, 16(1): 924-949. DOI: 10.3390/ijms16010924. [9] ARRANZ-NICOLÁS J, MÉRIDA I. Biological regulation of diacylglycerol kinases in normal and neoplastic tissues: New opportunities for cancer immunotherapy[J]. Adv Biol Regul, 2020, 75: 100663. DOI: 10.1016/j.jbior.2019.100663. [10] MÉRIDA I, TORRES-AYUSO P, ÁVILA-FLORES A, et al. Diacylglycerol kinases in cancer[J]. Adv Biol Regul, 2017, 63: 22-31. DOI: 10.1016/j.jbior.2016.09.005. [11] KAI M, YAMAMOTO E, SATO A, et al. Epigenetic silencing of diacylglycerol kinase gamma in colorectal cancer[J]. Mol Carcinog, 2017, 56(7): 1743-1752. DOI: 10.1002/mc.22631. [12] GUO Z, JIA J, YAO M, et al. Diacylglycerol kinase γ predicts prognosis and functions as a tumor suppressor by negatively regulating glucose transporter 1 in hepatocellular carcinoma[J]. Exp Cell Res, 2018, 373(1-2): 211-220. DOI: 10.1016/j.yexcr.2018.11.001. [13] HE Z, CHEN J, WANG J, et al. Expression of hepatitis B surface antigen in liver tissues can serve as a predictor of prognosis for hepatitis B virus-related hepatocellular carcinoma patients after liver resection[J]. Eur J Gastroenterol Hepatol, 2021, 33(1): 76-82. DOI: 10.1097/MEG.0000000000001698. [14] WANG L, LI Q, ZHANG J, et al. A novel prognostic scoring model based on albumin and γ-glutamyltransferase for hepatocellular carcinoma prognosis[J]. Cancer Manag Res, 2019, 11: 10685-10694. DOI: 10.2147/CMAR.S232073. [15] YAN X, YAO M, WEN X, et al. Elevated apolipoprotein B predicts poor postsurgery prognosis in patients with hepatocellular carcinoma[J]. Onco Targets Ther, 2019, 12: 1957-1964. DOI: 10.2147/OTT.S192631. [16] WU G, WU J, WANG B, et al. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: A population-based study[J]. Cancer Manag Res, 2018, 10: 4401-4410. DOI: 10.2147/CMAR.S177663. [17] WU XF, LIU YW, ZHANG H, et al. Application value of different Barcelona clinical liver cancer Kinki staging in radical resection of liver cancer[J]. Chin J Dig Surg, 2020, 19(12): 1266-1272. DOI: 10.3760/cma.j.cn115610-20201102-00691.吴晓峰, 刘一纬, 张慧, 等. 不同巴塞罗那临床肝癌Kinki分期在肝癌根治术中的应用价值[J]. 中华消化外科杂志, 2020, 19(12): 1266-1272. DOI: 10.3760/cma.j.cn115610-20201102-00691. [18] MASSART J, ZIERATH JR. Role of diacylglycerol kinases in glucose and energy homeostasis[J]. Trends Endocrinol Metab, 2019, 30(9): 603-617. DOI: 10.1016/j.tem.2019.06.003. [19] RAINERO E, CIANFLONE C, PORPORATO PE, et al. The diacylglycerol kinase α/atypical PKC/β1 integrin pathway in SDF-1α mammary carcinoma invasiveness[J]. PLoS One, 2014, 9(6): e97144. DOI: 10.1371/journal.pone.0097144. [20] FILIGHEDDU N, SAMPIETRO S, CHIANALE F, et al. Diacylglycerol kinase α mediates 17-β-estradiol-induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30[J]. Cell Signal, 2011, 23(12): 1988-1996. DOI: 10.1016/j.cellsig.2011.07.009. [21] DOMINGUEZ CL, FLOYD DH, XIAO A, et al. Diacylglycerol kinase α is a critical signaling node and novel therapeutic target in glioblastoma and other cancers[J]. Cancer Discov, 2013, 3(7): 782-797. DOI: 10.1158/2159-8290.CD-12-0215. [22] BACCHIOCCHI R, BALDANZI G, CARBONARI D, et al. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase[J]. Blood, 2005, 106(6): 2175-2182. DOI: 10.1182/blood-2005-01-0316. [23] KONG Y, ZHENG Y, JIA Y, et al. Decreased LIPF expression is correlated with DGKA and predicts poor outcome of gastric cancer[J]. Oncol Rep, 2016, 36(4): 1852-1860. DOI: 10.3892/or.2016.4989. [24] MCMURRAY HR, SAMPSON ER, COMPITELLO G, et al. Synergistic response to oncogenic mutations defines gene class critical to cancer phenotype[J]. Nature, 2008, 453(7198): 1112-1116. DOI: 10.1038/nature06973. [25] TAKEISHI K, TAKETOMI A, SHIRABE K, et al. Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEK-ERK pathway[J]. J Hepatol, 2012, 57(1): 77-83. DOI: 10.1016/j.jhep.2012.02.026. -




 PDF下载 ( 2110 KB)
PDF下载 ( 2110 KB)


 下载:
下载:

 下载:
下载:





