阿米替林对非酒精性脂肪性肝病细胞模型脂质沉积及生化代谢的影响
DOI: 10.3969/j.issn.1001-5256.2021.01.020
Effect of amitriptyline on lipid deposition and biochemical metabolism in a cell model of nonalcoholic fatty liver disease
-
摘要:
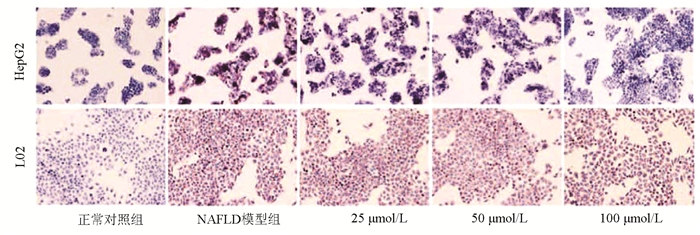
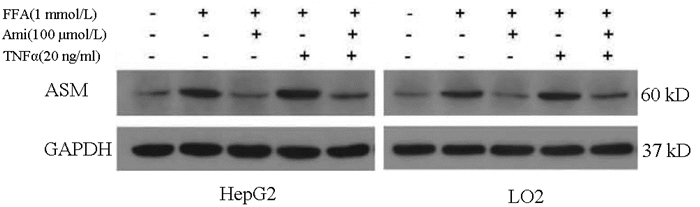
目的 探讨阿米替林通过调节酸性鞘磷脂酶(ASM)/神经酰胺(CE)通路对非酒精性脂肪性肝病(NAFLD)细胞模型脂质及生化代谢的影响。 方法 体外培养HepG2和L02细胞构建NAFLD细胞模型,MTT比色法测定细胞增殖率,油红O染色观察细胞内脂滴变化。实验分组:正常对照组、模型组、Ami组、TNFα组、Ami+TNFα组。全自动生化分析仪检测细胞内TG、TC及细胞上清液ALT、AST水平,ELISA法检测细胞内总的CE、ASM水平,Western Blot检测细胞内ASM蛋白的表达;实时荧光定量PCR检测细胞内ASM mRNA水平的表达。计量资料多组间比较采用单因素方差分析,进一步两两比较采用Turkey检验。 结果 与正常对照组相比,NAFLD模型组ASM蛋白和mRNA表达量以及CE、TG、TC、ALT、AST水平明显升高(P值均<0.05);与模型组相比,Ami组ASM蛋白和mRNA表达量以及CE、TG、TC、ALT、AST水平明显降低(P值均<0.05),TNFα组ASM蛋白和mRNA表达量以及CE、TG、ALT、AST水平明显升高(P值均<0.05);与TNFα组比较,Ami+TNFα组ASM蛋白和mRNA表达量以及CE、TG、TC、ALT、AST水平明显降低(P值均<0.05)。 结论 ASM/CE通路促进脂质积聚、导致脂肪变,阿米替林可通过抑制该通路改善NAFLD肝细胞的脂质沉积。 Abstract:Objective To investigate the effect of amitriptyline on lipid deposition and biochemical metabolism in a cell model of nonalcoholic fatty liver disease (NAFLD) by regulating the acid sphingomyelinase (ASM)/ceramide (CE) pathway. Methods HepG2 and L02 cells were cultured in vitro to establish a cell model of NAFLD. MTT colorimetry was used to measure cell proliferation rate, and oil red O staining was used to observe the change of lipid droplets in cells. In the experiment, the cells were divided into normal control group, model group, Ami group, TNFα group, and Ami+TNFα group. An automatic biochemical analyzer was used to measure the levels of triglyceride (TG) and total cholesterol (TC) in cells and the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in supernatant; ELISA was used to measure the levels of CE and ASM in cells; Western blot was used to measure the protein expression ASM in cells, and RT-PCR was used to measure the mRNA expression of ASM in cells. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the Turkey test was used for further comparison between two groups. Results Compared with the normal control group, the NAFLD model group had significant increases in the protein and mRNA expression of ASM and the levels of CE, TG, TC, ALT, and AST (all P < 0.05). Compared with the model group, the Ami group had significant reductions in the protein and mRNA expression of ASM and the levels of CE, TG, TC, ALT, and AST (all P < 0.05), and the TNFα group had significant increases in the protein and mRNA expression of ASM and the levels of CE, TG, ALT, and AST (all P < 0.05). Compared with the TNFα group, the Ami+TNFα group had significant reductions in the protein and mRNA expression of ASM and the levels of CE, TG, TC, ALT, and AST (all P < 0.05). Conclusion The ASM/CE pathway promotes lipid accumulation and may lead to hepatocyte steatosis, and amitriptyline can alleviate lipid deposition in NAFLD hepatocytes by inhibiting the ASM/CE pathway. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- Amitriptyline /
- PhospHolipases /
- Ceramides /
- Lipidoses
-
表 1 不同浓度FFA干预细胞的OD值和细胞增殖率
FFA浓度(mmol/L) HepG2 L02 OD值 增殖率(%) OD值 增殖率(%) 0 0.755±0.038 100.00 0.861±0.046 100.00 0.125 0.744±0.038 98.45 0.872±0.028 101.37 0.25 0.727±0.030 95.90 0.838±0.027 97.05 0.5 0.676±0.038 88.20 0.839±0.032 97.17 1 0.645±0.0441) 83.60 0.729±0.033 91.05 2 0.561±0.0121) 71.00 0.740±0.0251) 84.38 F值 13.472 6.930 P值 <0.05 <0.05 注:1)与空白组比较,P<0.05。 表 2 正常对照组和NAFLD模型组细胞内TG、TC、FFA含量比较
组别 HepG2 L02 TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) 正常对照组 0.142±0.011 0.171±0.033 55.009±6.285 0.117±0.013 0.151±0.018 44.906±4.881 NAFLD模型组 0.541±0.056 0.633±0.045 224.585±25.600 0.370±0.055 0.458±0.052 196.491±14.846 t值 10.398 10.749 9.215 6.64 8.799 15.362 P值 0.009 0.009 0.012 0.022 0.013 0.004 表 3 不同浓度阿米替林干预脂肪变细胞的OD值和增殖率
组别 HepG2 L02 OD值 增殖率(%) OD值 增殖率(%) 正常对照组 0.814±0.045 100.00 0.950±0.027 100.00 NAFLD模型组 0.727±0.040 88.11 0.892±0.043 93.34 Ami 25 μmol/L 0.741±0.040 90.08 0.887±0.026 92.80 Ami 50 μmol/L 0.755±0.036 91.91 0.907±0.031 95.07 Ami 100 μmol/L 0.778±0.040 95.06 0.930±0.023 97.73 F值 2.139 2.188 P值 >0.05 >0.05 表 4 各组HepG2细胞内TG、TC、FFA水平比较
组别 TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) 正常对照组 0.153±0.010 0.170±0.012 65.350±11.407 NAFLD模型组 0.566±0.0301) 0.631±0.0431) 266.266±18.1121) Ami 25 mmol/L 0.553±0.029 0.622±0.020 268.887±8.053 Ami 50 mmol/L 0.362±0.0542) 0.506±0.0422) 204.097±11.2802) Ami 100 mmol/L 0.233±0.0202) 0.342±0.0242) 166.003±12.8372) F值 99.124 124.967 125.931 P值 <0.05 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05。 表 5 各组L02细胞内TG、TC、FFA水平比较
组别 TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) 正常对照组 0.127±0.015 0.139±0.012 48.600±6.942 NAFLD模型组 0.384±0.0301) 0.504±0.0441) 212.226±13.0891) Ami 25 mmol/L 0.363±0.016 0.458±0.016 210.774±14.166 Ami 50 mmol/L 0.254±0.0192) 0.380±0.0262) 152.150±8.9002) Ami 100 mmol/L 0.205±0.0302) 0.282±0.0252) 122.543±11.2842) F值 66.778 90.103 111.315 P值 <0.05 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05。 表 6 各组细胞ASM的mRNA相对表达量
组别 HepG2 L02 正常对照组 1 1 NAFLD模型组 2.069±0.1241) 1.847±0.3861) Ami组 1.462±0.3792) 1.321±0.364 TNFα组 2.685±0.3672) 2.708±0.1952) Ami+TNFα组 1.888±0.4933) 1.754±0.3733) F值 11.28 13.653 P值 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05;3)与TNFα组比较,P<0.05。 表 7 各组细胞的ELISA检测结果(OD值)
组别 HepG2 L02 ASM CE ASM CE 正常对照组 0.338±0.004 0.104±0.003 0.312±0.005 0.100±0.002 NAFLD模型组 0.667±0.0131) 0.174±0.0031) 0.571±0.0031) 0.157±0.0031) Ami组 0.343±0.0062) 0.116±0.0062) 0.331±0.0052) 0.114±0.0052) TNFα组 0.791±0.0042) 0.220±0.0042) 0.670±0.0022) 0.187±0.0042) Ami+TNFα组 0.453±0.0033) 0.130±0.0033) 0.393±0.0043) 0.120±0.0073) F值 2594.967 450.127 5098.961 194.498 P值 <0.05 <0.05 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05;3)与TNFα组比较,P<0.05。 表 8 各组HepG2细胞的生化指标比较
组别 TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) ALT(U/L) AST(U/L) 正常对照组 0.159±0.022 0.169±0.013 63.498±6.788 7.701±0.516 6.379±0.865 NAFLD模型组 0.534±0.0711) 0.640±0.0681) 255.506±28.8821) 28.566±2.7211) 23.558±4.0831) Ami组 0.209±0.2172) 0.194±0.0212) 123.956±12.2642) 9.247±1.5132) 8.068±1.6232) TNFα组 0.720±0.0552) 0.729±0.033 302.303±17.7382) 34.552±3.9052) 29.409±3.0222) Ami+TNFα组 0.262±0.0413) 0.303±0.0663) 166.610±17.6083) 14.604±3.5073) 10.272±1.6863) F值 80.053 95.042 85.008 57.547 49.669 P值 <0.05 <0.05 <0.05 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05;3)与TNFα组比较,P<0.05。 表 9 各组L02细胞的生化指标比较
组别 TG(mmol/g prot) TC(mmol/g prot) FFA(mmol/g prot) ALT(U/L) AST(U/L) 正常对照组 0.130±0.015 0.141±0.011 50.665±5.181 6.154±0.757 7.131±0.948 NAFLD模型组 0.402±0.0401) 0.503±0.0301) 211.240±17.3701) 12.201±3.1561) 18.214±2.1531) Ami组 0.157±0.0202) 0.171±0.0182) 106.681±11.5492) 6.231±0.8572) 8.274±1.4392) TNFα组 0.553±0.0382) 0.615±0.0642) 271.230±12.7982) 18.152±1.8062) 25.549±2.9922) Ami+TNFα组 0.213±0.0233) 0.234±0.0253) 145.189±18.3773) 7.917±1.5383) 9.271±1.6943) F值 116.301 113.124 116.704 23.211 48.842 P值 <0.05 <0.05 <0.05 <0.05 <0.05 注:1)与正常对照组比较,P<0.05;2)与NAFLD模型组比较,P<0.05;3)与TNFα组比较,P<0.05。 -
[1] MUNDI MS, VELAPATI S, PATEL J, et al. Evolution of NAFLD and its management[J]. Nutr Clin Pract, 2020, 35(1): 72-84. DOI: 10.1002/ncp.10449 [2] JIANG YZ, NIE HM, WANG R. Research advances in the pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2019, 35(11): 2588-2591. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.11.044姜煜资, 聂红明, 汪蓉. 非酒精性脂肪性肝病的发病机制[J]. 临床肝胆病杂志, 2019, 35(11): 2588-2591. DOI: 10.3969/j.issn.1001-5256.2019.11.044 [3] BECKMANN N, SHARMA D, GULBINS E, et al. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons[J]. Front Physiol, 2014, 5: 331. http://www.ncbi.nlm.nih.gov/pubmed/25228885 [4] YANG L, JIN GH, ZHOU JY. The role of ceramide in the pathogenesis of alcoholic liver disease[J]. Alcohol Alcohol, 2016, 51(3): 251-257. DOI: 10.1093/alcalc/agv119 [5] RÉGNIER M, POLIZZI A, GUILLOU H, et al. Sphingolipid metabolism in non-alcoholic fatty liver diseases[J]. Biochimie, 2019, 159: 9-22. DOI: 10.1016/j.biochi.2018.07.021 [6] UNGER RH. Lipotoxic diseases[J]. Annu Rev Med, 2002, 53(4): 319-336. [7] MALDONADO-HERNÁNDEZ J, SALDAÑA-DÁVILA GE, PIÑA-AGUERO MI, et al. Association between plasmatic ceramides profile and AST/ALT Ratio: C14:0 ceramide as predictor of hepatic steatosis in adolescents independently of obesity[J]. Can J Gastroenterol Hepatol, 2017, 2017: 3689375. [8] APOSTOLOPOULOU M, GORDILLO R, KOLIAKI C, et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis[J]. Diabetes Care, 2018, 41(6): 1235-1243. DOI: 10.2337/dc17-1318 [9] LUUKKONEN PK, ZHOU Y, SÄDEVIRTA S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64(5): 1167-1175. DOI: 10.1016/j.jhep.2016.01.002 [10] HU Y, LIU ZX, FU N, et al. Role of ceramide in hepatic lipid accumulation in rats with non-alcoholic fatty liver disease[J]. World Chin J Dig, 2015, 23(32): 5196-5200. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-XXHB201532019.htm胡杨, 刘朝霞, 傅念, 等. 神经酰胺在非酒精性脂肪肝大鼠肝细胞脂质沉积中的作用[J]. 世界华人消化杂志, 2015, 23(32): 5196-5200. https://www.cnki.com.cn/Article/CJFDTOTAL-XXHB201532019.htm [11] FERNANDEZ A, MATIAS N, FUCHO R, et al. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading[J]. J Hepatol, 2013, 59(4): 805-813. DOI: 10.1016/j.jhep.2013.05.023 [12] FUCHO R, MARTÍNEZ L, BAULIES A, et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis[J]. J Hepatol, 2014, 61(5): 1126-1134. DOI: 10.1016/j.jhep.2014.06.009 [13] LIANGPUNSAKUL S, RAHMINI Y, ROSS RA, et al. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(5): g515-g523. DOI: 10.1152/ajpgi.00455.2011 [14] GARCIA-RUIZ C, MATO JM, VANCE D, et al. Acid sphingomyelinase-ceramide system in steatohepatitis: A novel target regulating multiple pathways[J]. J Hepatol, 2015, 62(1): 219-233. DOI: 10.1016/j.jhep.2014.09.023 [15] MVHLE C, WEINLAND C, GULBINS E, et al. Peripheral acid sphingomyelinase activity is associated with biomarkers and phenotypes of alcohol use and dependence in patients and healthy controls[J]. Int J Mol Sci, 2018, 19(12): 4028. DOI: 10.3390/ijms19124028 [16] GUAN Y, LI X, UMETANI M, et al. Tricyclic antidepressant amitriptyline inhibits autophagic flux and prevents tube formation in vascular endothelial cells[J]. Basic Clin Pharmacol Toxicol, 2019, 124(4): 370-384. DOI: 10.1111/bcpt.13146 [17] LU Z, LI Y, SYN WK, et al. Amitriptyline inhibits nonalcoholic steatohepatitis and atherosclerosis induced by high-fat diet and LPS through modulation of sphingolipid metabolism[J]. Am J Physiol Endocrinol Metab, 2020, 318(2): e131-e144. DOI: 10.1152/ajpendo.00181.2019 -



 PDF下载 ( 2482 KB)
PDF下载 ( 2482 KB)


 下载:
下载: