胆管癌和胆总管结石患者胆汁中钙卫蛋白检测的临床意义
DOI: 10.12449/JCH240321
Clinical significance of determining the level of biliary calprotectin in patients with cholangiocarcinoma or choledocholithiasis
-
摘要:
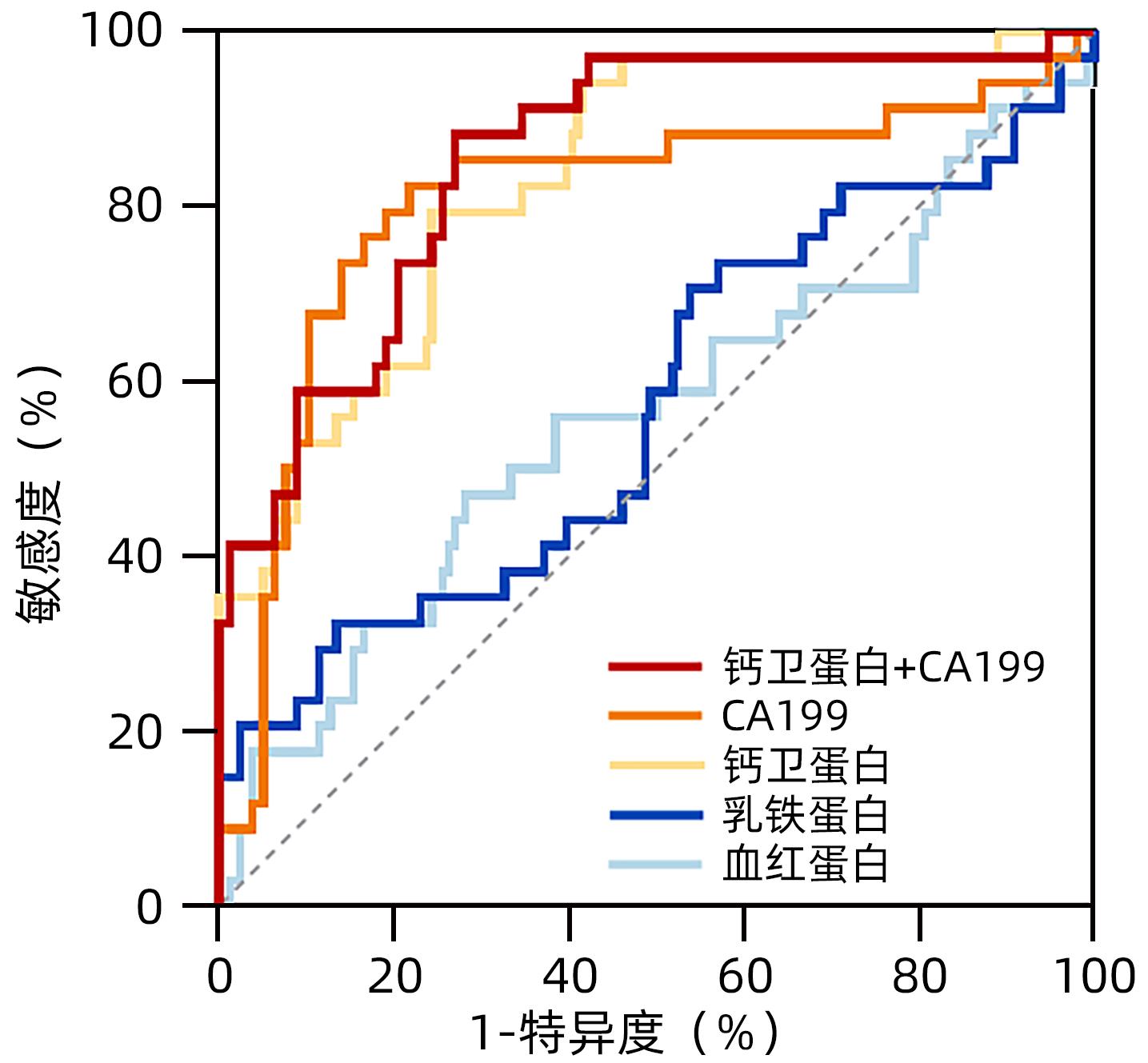
目的 探讨胆管癌和胆总管结石患者胆汁中钙卫蛋白水平的差异。 方法 收集2021年5月—2022年9月安徽医科大学第一附属医院行ERCP诊治的胆管癌(n=34)和胆总管结石(n=78)患者的临床资料和胆汁标本。采用荧光免疫层析法检测胆汁中钙卫蛋白、血红蛋白和乳铁蛋白水平。计量资料两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验;相关分析采用Spearman相关性检验;DeLong检验比较不同受试者工作特征曲线(ROC曲线)下面积(AUC)的差异。 结果 与胆总管结石组比较,胆管癌患者胆汁中钙卫蛋白水平升高[4 795.50(2 286.79~20 179.73)ng/mL vs 411.16(67.03~1 991.88)ng/mL,Z=5.572,P<0.001],同时氯化物水平升高[115.70(109.10~125.50)mmol/L vs 106.60(98.60~114.40)mmol/L,Z=2.702,P=0.007]。进一步将胆管癌分为高位胆管癌和低位胆管癌,两组钙卫蛋白比较差异无统计学意义[3 867.71(2 235.66~26 407.40)ng/mL vs 4 795.50(2 361.15~13 070.53)ng/mL,Z=0.129,P>0.05]。胆汁钙卫蛋白水平与胆汁白细胞计数、血红蛋白、乳铁蛋白水平具有相关性(r值分别为0.316、0.353、0.464,P值均<0.05)。ROC曲线结果示胆汁钙卫蛋白(敏感度79.4%,特异度75.6%)、血CA19-9水平(敏感度82.4%,特异度78.2%)以及两者联合(敏感度88.2%,特异度73.1%)对诊断胆管癌具有良好的敏感性和特异性。 结论 胆管癌患者胆汁中钙卫蛋白水平升高,可能成为胆管癌诊断的生物标志物。 -
关键词:
- 胆管肿瘤 /
- 胆总管结石病 /
- 白细胞L1抗原复合物
Abstract:Objective To investigate the difference in the level of biliary calprotectin between patients with cholangiocarcinoma and those with choledocholithiasis. Methods Clinical data and bile samples were collected from 34 patients with cholangiocarcinoma and 78 patients with choledocholithiasis who were diagnosed and treated with endoscopic retrograde cholangiopancreatography in The First Affiliated Hospital of Anhui Medical University from May 2021 to September 2022. Fluorescence lateral flow immunoassay was used to measure the levels of calprotectin, hemoglobin, and lactoferrin in bile. The Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups; the Spearman correlation test was used for correlation analysis; the DeLong test was used for comparison of the area under the ROC curve (AUC). Results Compared with the choledocholithiasis group, the cholangiocarcinoma group had significant increases in the levels of calprotectin [4 795.50 (2 286.79 — 20 179.73) ng/mL vs 411.16 (67.03 — 1 991.88) ng/mL, Z=5.572, P<0.001] and fluoride [115.70 (109.10 — 125.50) mmol/L vs 106.60 (98.60 — 114.40) mmol/L, Z=2.702, P=0.007]. The patients with cholangiocarcinoma were further divided into high cholangiocarcinoma group and low cholangiocarcinoma group, and there was no significant difference between the two groups in the level of calprotectin [3 867.71 (2 235.66 — 26 407.40) ng/mL vs 4 795.50 (2 361.15 — 13 070.53) ng/mL, Z=0.129, P>0.05]. Biliary calprotectin level was correlated with white blood cell count, hemoglobin concentration, and lactoferrin concentration in bile (r=0.316, 0.353, and 0.464, all P<0.05). The ROC curve analysis showed that biliary calprotectin (with a sensitivity of 79.4% and a specificity of 75.6%), blood CA19-9 (with a sensitivity of 82.4% and a specificity of 78.2%), and their combination (with a sensitivity of 88.2% and a specificity of 73.1%) had good sensitivity and specificity in the diagnosis of cholangiocarcinoma. Conclusion There is an increase in the level of biliary calprotectin in patients with cholangiocarcinoma, and therefore, it might become a biomarker for the diagnosis of cholangiocarcinoma. -
Key words:
- Bile Duct Neoplasms /
- Choledocholithiasis /
- Leukocyte L1 Antigen Complex
-
表 1 胆管癌组与胆总管结石组患者临床资料比较
Table 1. Clinical data of patients with cholangiocarcinoma and cholangiolithiasis
项目 胆总管结石组(n=78) 胆管癌组(n=34) 统计值 P值 男/女(例) 40/38 19/15 χ2=0.201 0.654 年龄(岁) 68.50(56.00~77.25) 73.50(69.00~80.00) Z=2.321 0.020 WBC(×109/L) 5.84(4.65~7.78) 7.51(5.38~10.16) Z=2.082 0.037 NEU(%) 66.50(50.73~77.75) 79.70(68.35~89.35) Z=4.028 <0.001 血红蛋白(g/L) 122.00±14.76 102.76±18.25 Z=5.169 <0.001 Alb(g/L) 38.33±4.70 32.33±5.31 Z=5.044 <0.001 TBil(μmol/L) 19.59(12.70~42.40) 152.13(49.18~271.88) Z=5.588 <0.001 ALT(U/L) 46.50(21.75~150.50) 88.00(61.50~194.75) Z=2.721 0.006 AST(U/L) 35.00(23.00~97.75) 124.00(66.50-220.75) Z=4.531 <0.001 ALP(U/L) 154.00(94.00~248.25) 627.00(330.25~1 019.25) Z=6.056 <0.001 AFP(ng/mL) 2.08(1.38~3.04) 1.87(1.30~2.86) Z=0.940 0.347 CEA(ng/mL) 1.46(0.73~2.53) 2.36(1.46~6.16) Z=3.163 0.002 CA19-9(U/mL) 17.80(8.66~47.80) 342.34(127.99~1 000.00) Z=5.126 <0.001 表 2 胆管癌组与胆总管结石组患者胆汁常规和生化检查结果
Table 2. Routine and biochemical examination of bile in patients with cholangiocarcinoma and cholangiolithiasis
项目 胆总管结石组(n=78) 胆管癌组(n=34) Z值 P值 WBC(×106/L) 7.00(2.00~59.00) 22.50(2.75~60.50) 0.847 0.397 RBC(×106/L) 0.00(0.00~58.00) 17.00(0.00~637.50) 1.473 0.141 多个核细胞百分比(%) 70.40(28.60~100.00) 71.40(42.50~93.10) 0.297 0.766 氯化物(mmol/L) 106.60(98.60~114.40) 115.70(109.10~125.50) 2.702 0.007 葡萄糖(mmol/L) 0.07(0.01~0.45) 0.01(0.01~0.14) 1.254 0.210 蛋白(g/L) 0.15(0.10~3.25) 1.00(0.10~4.30) 1.206 0.228 LDH(U/L) 73.50(36.50~157.50) 99.00(58.00~437.00) 1.273 0.203 表 3 胆管癌组与胆总管结石组患者胆汁钙卫蛋白等水平检测
Table 3. Determination of calprotectin and other substances in bile of cholangiocarcinoma and cholangiolithiasis patients
项目 胆总管结石组(n=78) 胆管癌组(n=34) Z值 P值 钙卫蛋白(ng/mL) 411.16(67.03~1 991.88) 4 795.50(2 286.79~20 179.73) 5.572 <0.001 血红蛋白(ng/mL) 6 338.13(1 430.09~27 453.84) 14 100.82(713.08~44 695.15) 1.006 0.314 乳铁蛋白(ng/mL) 478.79(184.75~1 319.59) 523.19(224.53~3 526.59) 1.174 0.240 表 4 胆管癌患者胆汁中钙卫蛋白等指标的预测作用
Table 4. Predictive effect of calprotectin level in bile of patients with cholangiocarcinoma
项目 AUC P值 截断值 敏感度 特异度 阳性预测值 阴性预测值 钙卫蛋白 0.832 <0.001 1 977.17 0.794 0.756 0.587 0.894 血红蛋白 0.560 0.314 19 001.22 0.471 0.718 乳铁蛋白 0.570 0.240 2 258.57 0.324 0.859 血CA19-9 0.805 <0.001 55.47 0.824 0.782 0.622 0.910 钙卫蛋白+CA19-9 0.855 <0.001 0.19 0.882 0.731 0.588 0.934 -
[1] RAGGI C, TADDEI ML, RAE C, et al. Metabolic reprogramming in cholangiocarcinoma[J]. J Hepatol, 2022, 77( 3): 849- 864. DOI: 10.1016/j.jhep.2022.04.038. [2] JANG S, STEVENS T, KOU L, et al. Efficacy of digital single-operator cholangioscopy and factors affecting its accuracy in the evaluation of indeterminate biliary stricture[J]. Gastrointest Endosc, 2020, 91( 2): 385- 393.e1. DOI: 10.1016/j.gie.2019.09.015. [3] CHEN S, WANG J. Advances in tumor microenvironment and immunotherapy of cholangiocarcinoma[J]. J Clin Hepatol, 2022, 38( 10): 2428- 2432. DOI: 10.3969/j.issn.1001-5256.2022.10.044.陈顺, 王俊. 胆管癌肿瘤微环境与免疫治疗[J]. 临床肝胆病杂志, 2022, 38( 10): 2428- 2432. DOI: 10.3969/j.issn.1001-5256.2022.10.044. [4] ZUO S, CHEN Q, ZOU WL. Current status and prospect of immunotherapy for cholangiocarcinoma[J]. Chin J Dig Surg, 2022, 21( 7): 873- 879. DOI: 10.3760/cma.j.cn115610-20220506-00254.左石, 陈乾, 邹卫龙. 胆管癌免疫治疗的现状与展望[J]. 中华消化外科杂志, 2022, 21( 7): 873- 879. DOI: 10.3760/cma.j.cn115610-20220506-00254. [5] GIESE MA, HIND LE, HUTTENLOCHER A. Neutrophil plasticity in the tumor microenvironment[J]. Blood, 2019, 133( 20): 2159- 2167. DOI: 10.1182/blood-2018-11-844548. [6] JUKIC A, BAKIRI L, WAGNER EF, et al. Calprotectin: From biomarker to biological function[J]. Gut, 2021, 70( 10): 1978- 1988. DOI: 10.1136/gutjnl-2021-324855. [7] ARGYRIS PP, SLAMA ZM, ROSS KF, et al. Calprotectin and the initiation and progression of head and neck cancer[J]. J Dent Res, 2018, 97( 6): 674- 682. DOI: 10.1177/0022034518756330. [8] SHABANI F, FARASAT A, MAHDAVI M, et al. Calprotectin(S100A8/S100A9): A key protein between inflammation and cancer[J]. Inflamm Res, 2018, 67( 10): 801- 812. DOI: 10.1007/s00011-018-1173-4. [9] PAN SG, HU Y, HU MJ, et al. S100A8 facilitates cholangiocarcinoma metastasis via upregulation of VEGF through TLR4/NF-κB pathway activation[J]. Int J Oncol, 2020, 56( 1): 101- 112. DOI: 10.3892/ijo.2019.4907. [10] LIANG HJ, QIN SK, SHEN F, et al. Expert consensus on diagnosis and treatment of CSCO biliary systerm tumors(2019 edition)[J]. Chin Clin Oncol, 2019, 24( 9): 828- 838.梁后杰, 秦叔逵, 沈锋, 等. CSCO胆道系统肿瘤诊断治疗专家共识(2019年版)[J]. 临床肿瘤学杂志, 2019, 24( 9): 828- 838. [11] DELONG ER, DELONG DM, CLARKE-PEARSON DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach[J]. Biometrics, 1988, 44( 3): 837- 845. [12] KHAN SA, DAVIDSON BR, GOLDIN RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update[J]. Gut, 2012, 61( 12): 1657- 1669. DOI: 10.1136/gutjnl-2011-301748. [13] CAO HS, HUANG T, DAI MR, et al. Tumor microenvironment and its implications for antitumor immunity in cholangiocarcinoma: Future perspectives for novel therapies[J]. Int J Biol Sci, 2022, 18( 14): 5369- 5390. DOI: 10.7150/ijbs.73949. [14] MAO ZY, ZHU GQ, XIONG M, et al. Prognostic value of neutrophil distribution in cholangiocarcinoma[J]. World J Gastroenterol, 2015, 21( 16): 4961- 4968. DOI: 10.3748/wjg.v21.i16.4961. [15] ZHOU SL, DAI Z, ZHOU ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils[J]. Carcinogenesis, 2014, 35( 3): 597- 605. DOI: 10.1093/carcin/bgt397. [16] LI YW, QIU SJ, FAN J, et al. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection[J]. J Hepatol, 2011, 54( 3): 497- 505. DOI: 10.1016/j.jhep.2010.07.044. [17] JENSEN HK, DONSKOV F, MARCUSSEN N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma[J]. J Clin Oncol, 2009, 27( 28): 4709- 4717. DOI: 10.1200/JCO.2008.18.9498. [18] JAILLON S, PONZETTA A, DI MITRI D, et al. Neutrophil diversity and plasticity in tumour progression and therapy[J]. Nat Rev Cancer, 2020, 20( 9): 485- 503. DOI: 10.1038/s41568-020-0281-y. [19] SHAUL ME, FRIDLENDER ZG. Tumour-associated neutrophils in patients with cancer[J]. Nat Rev Clin Oncol, 2019, 16( 10): 601- 620. DOI: 10.1038/s41571-019-0222-4. [20] VOIGTLÄNDER T, WLECKE J, NEGM AA, et al. Calprotectin in bile: A disease severity marker in patients with primary sclerosing cholangitis[J]. J Clin Gastroenterol, 2014, 48( 10): 866- 869. DOI: 10.1097/MCG.0000000000000042. [21] GAUSS A, SAUER P, STIEHL A, et al. Evaluation of biliary calprotectin as a biomarker in primary sclerosing cholangitis[J]. Medicine, 2016, 95( 17): e3510. DOI: 10.1097/MD.0000000000003510. [22] SRIKRISHNA G. S100A8 and S100A9: New insights into their roles in malignancy[J]. J Innate Immun, 2012, 4( 1): 31- 40. DOI: 10.1159/000330095. [23] JAMNONGKAN W, THANAN R, TECHASEN A, et al. Upregulation of transferrin receptor-1 induces cholangiocarcinoma progression via induction of labile iron pool[J]. Tumour Biol, 2017, 39( 7): 1010428317717655. DOI: 10.1177/1010428317717655. [24] MANCINELLI R, CUTONE A, ROSA L, et al. Different iron-handling in inflamed small and large cholangiocytes and in small and large-duct type intrahepatic cholangiocarcinoma[J]. Eur J Histochem, 2020, 64( 4): 3156. DOI: 10.4081/ejh.2020.3156. -



 PDF下载 ( 643 KB)
PDF下载 ( 643 KB)

 下载:
下载:


