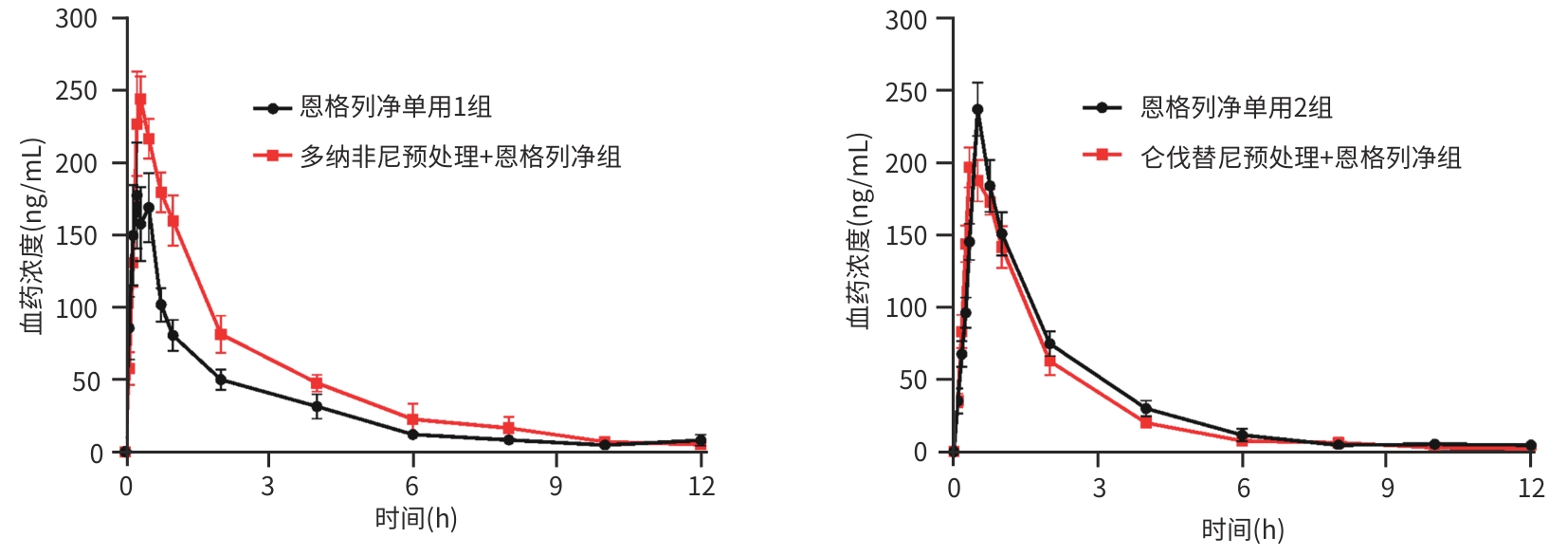
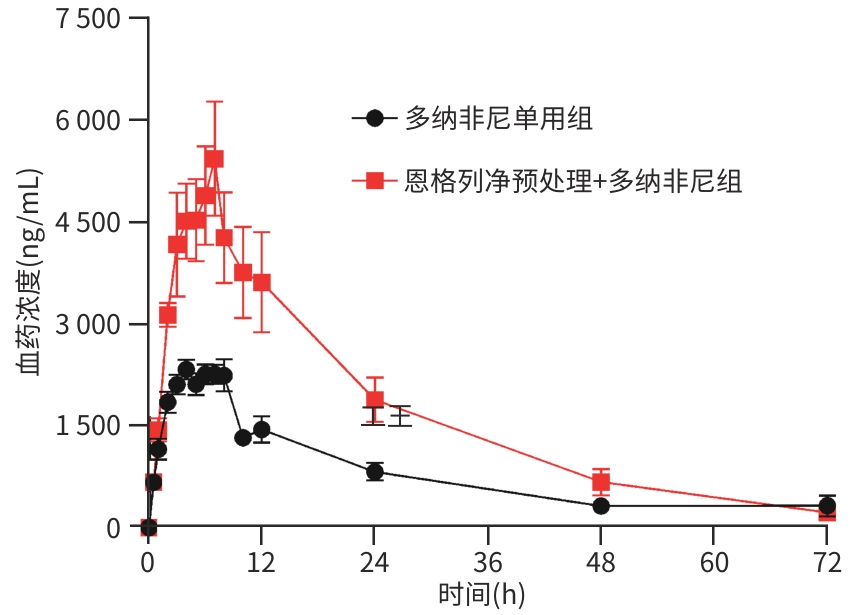
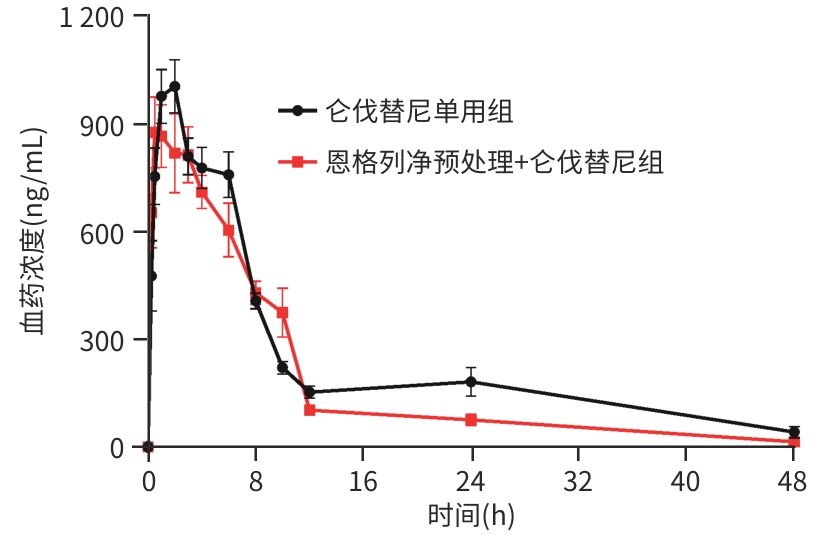
| [1] |
|
| [2] |
GUPTA A, JARZAB B, CAPDEVILA J, et al. Population pharmacokinetic analysis of lenvatinib in healthy subjects and patients with cancer[J]. Br J Clin Pharmacol, 2016, 81( 6): 1124- 1133. DOI: 10.1111/bcp.12907. |
| [3] |
HE XR, LI Y, LI YJ, et al. In vivo assessment of the pharmacokinetic interactions between donafenib and dapagliflozin, donafenib and canagliflozin in rats[J]. Biomed Pharmacother, 2023, 162: 114663. DOI: 10.1016/j.biopha.2023.114663. |
| [4] |
MIRARCHI L, AMODEO S, CITARRELLA R, et al. SGLT2 inhibitors as the most promising influencers on the outcome of non-alcoholic fatty liver disease[J]. Int J Mol Sci, 2022, 23( 7): 3668. DOI: 10.3390/ijms23073668. |
| [5] |
LIU ZH, DU WY, GUO CH, et al. Research progress of empagliflozin in the treatment of type 2 diabe-tes mellitus and cardiovascular and renal benefits[J]. Chin J Clin Pharmacol Ther, 2025, 30( 3): 412- 418. DOI: 10.12092/j.issn.1009-2501.2025.03.015. |
| [6] |
STÖLLBERGER C, FINSTERER J, SCHNEIDER B. Adverse events and drug-drug interactions of sodium glucose co-transporter 2 inhibitors in patients treated for heart failure[J]. Expert Rev Cardiovasc Ther, 2023, 21( 11): 803- 816. DOI: 10.1080/14779072.2023.2273900. |
| [7] |
CUI YJ, LI Y, FAN LJ, et al. UPLC-MS/MS method for the determination of Lenvatinib in rat plasma and its application to drug-drug interaction studies[J]. J Pharm Biomed Anal, 2021, 206: 114360. DOI: 10.1016/j.jpba.2021.114360. |
| [8] |
Chinese Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China(PRC)-part IV(2020 edition)[M]. Beijing: China Medical Science Press, 2020.
国家药典委员会. 中华人民共和国药典-四部(2020年版)[M]. 北京: 中国医药科技出版社, 2020.
|
| [9] |
SCHEEN AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor[J]. Clin Pharmacokinet, 2014, 53( 3): 213- 225. DOI: 10.1007/s40262-013-0126-x. |
| [10] |
GARCIA-ROPERO A, BADIMON JJ, SANTOS-GALLEGO CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: The latest developments[J]. Expert Opin Drug Metab Toxicol, 2018, 14( 12): 1287- 1302. DOI: 10.1080/17425255.2018.1551877. |
| [11] |
|
| [12] |
REAGAN-SHAW S, NIHAL M, AHMAD N. Dose translation from animal to human studies revisited[J]. FASEB J, 2008, 22( 3): 659- 661. DOI: 10.1096/fj.07-9574LSF. |
| [13] |
MACHA S, KOENEN R, SENNEWALD R, et al. Effect of gemfibrozil, rifampicin, or probenecid on the pharmacokinetics of the SGLT2 inhibitor empagliflozin in healthy volunteers[J]. Clin Ther, 2014, 36( 2): 280- 290. e 1. DOI: 10.1016/j.clinthera.2014.01.003. |
| [14] |
DENG YR, CAO GX, YAN B, et al. Effect of sorafenib and donafenib on the pharmacokinetics of ertugliflozin in rats[J]. J Clin Hepatol, 2025, 41( 1): 92- 98. DOI: 10.12449/JCH250114. 邓艳茹, 曹格溪, 闫彬, 等. 索拉非尼和多纳非尼对大鼠体内艾托格列净药代动力学的影响[J]. 临床肝胆病杂志, 2025, 41( 1): 92- 98. DOI: 10.12449/JCH250114. |
| [15] |
WANG XM, ZHANG X, HUANG XH, et al. The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4[J]. Xenobiotica, 2016, 46( 7): 651- 658. DOI: 10.3109/00498254.2015.1109160. |
| [16] |
LI JM, WANG XQ, NING C, et al. Influences of ABC transporter and CYP3A4/5 genetic polymorphisms on the pharmacokinetics of lenvatinib in Chinese healthy subjects[J]. Eur J Clin Pharmacol, 2020, 76( 8): 1125- 1133. DOI: 10.1007/s00228-020-02879-z. |
| [17] |
SHUMAKER R, ALURI J, FAN J, et al. Effects of ketoconazole on the pharmacokinetics of lenvatinib(E7080) in healthy participants[J]. Clin Pharmacol Drug Dev, 2015, 4( 2): 155- 160. DOI: 10.1002/cpdd.140. |
| [18] |
SHUMAKER RC, ALURI J, FAN J, et al. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults[J]. Clin Drug Investig, 2014, 34( 9): 651- 659. DOI: 10.1007/s40261-014-0217-y. |







 DownLoad:
DownLoad: