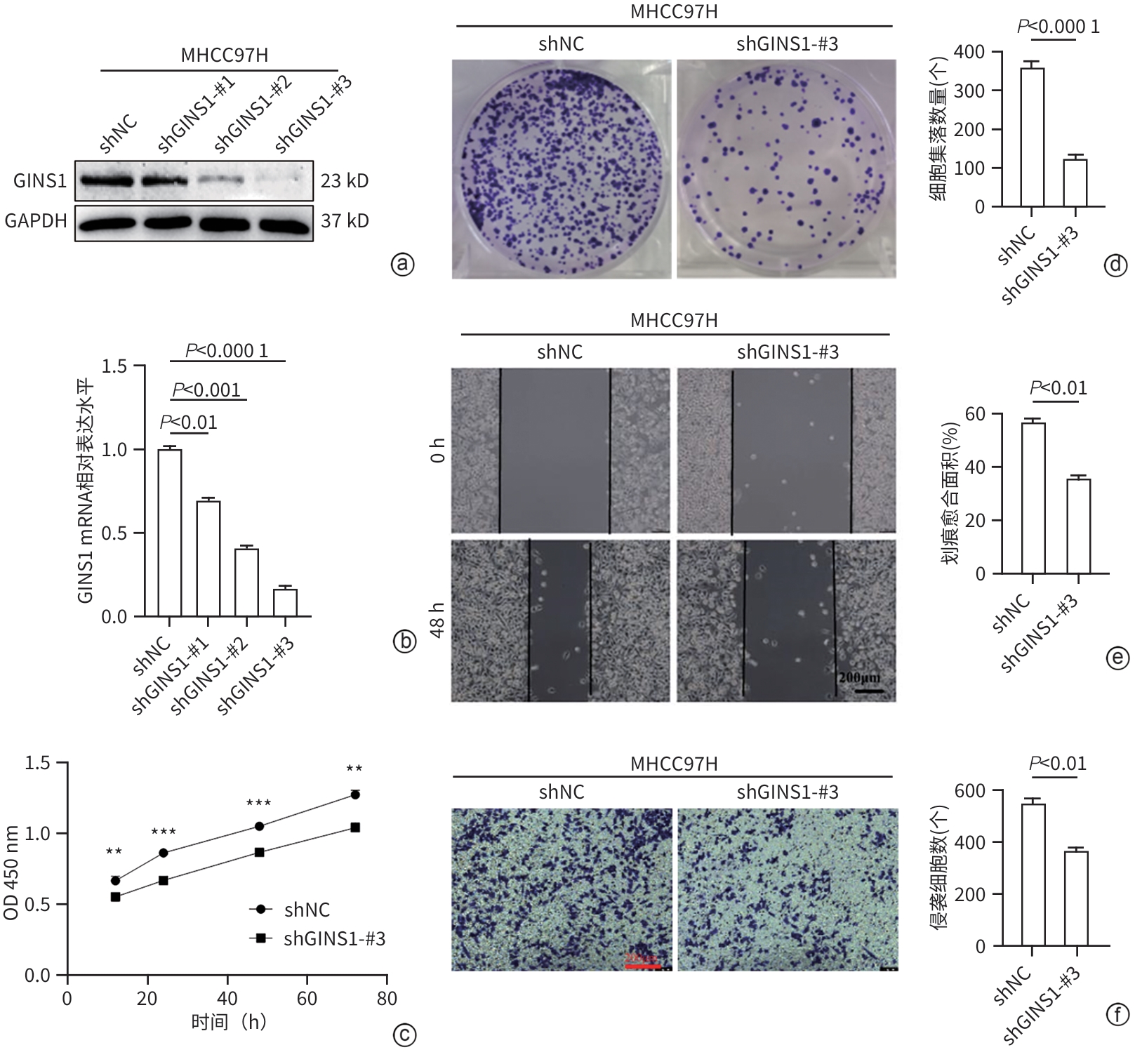
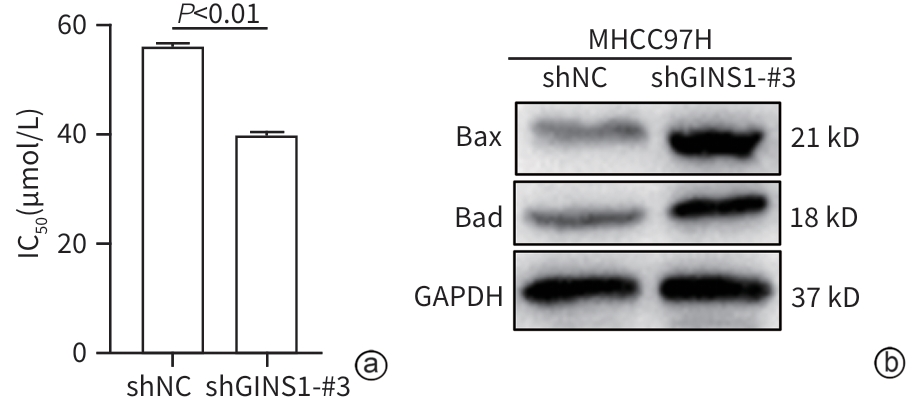
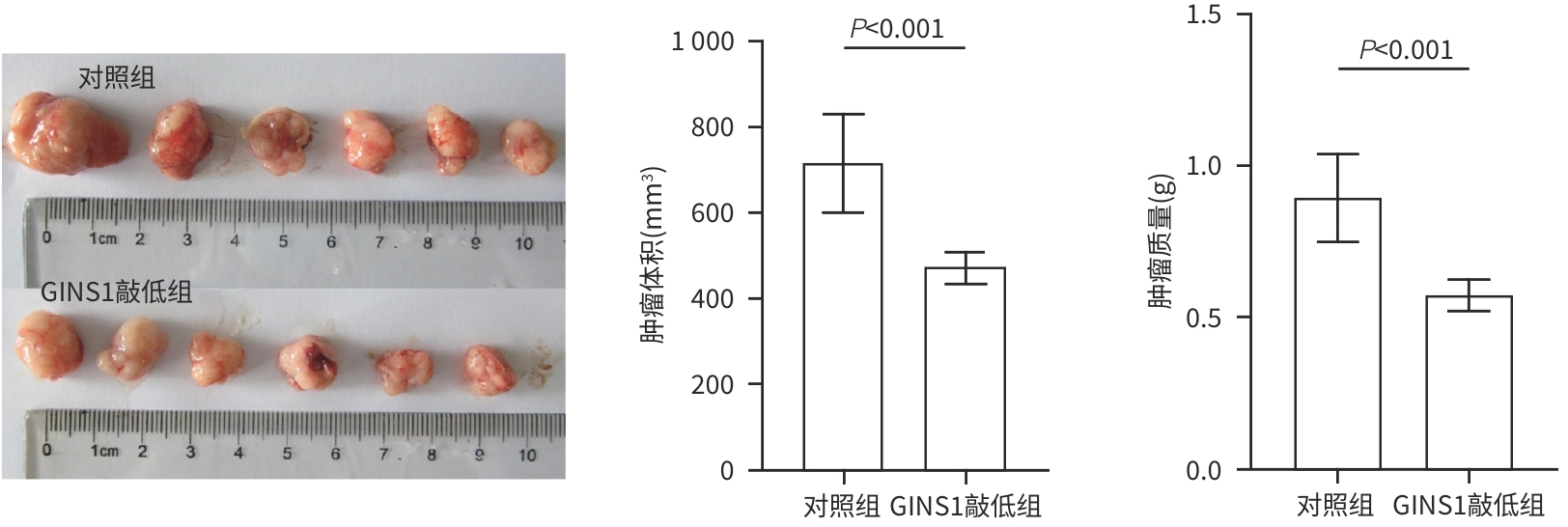
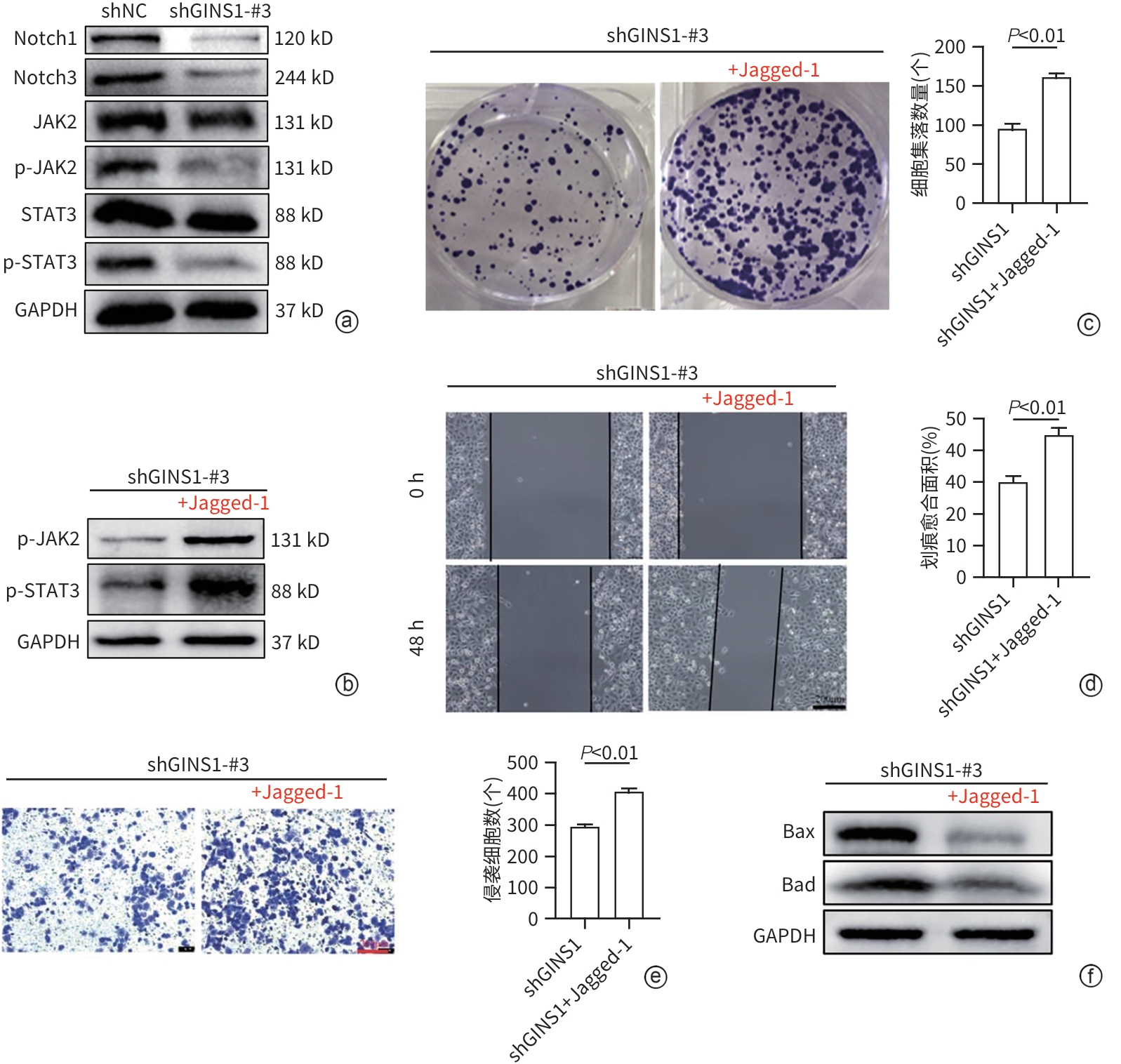
| [1] |
WANG Y, DENG BC. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers[J]. Cancer Metastasis Rev, 2023, 42( 3): 629- 652. DOI: 10.1007/s10555-023-10084-4. |
| [2] |
National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. 中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. |
| [3] |
WANG WY, WEI C. Advances in the early diagnosis of hepatocellular carcinoma[J]. Genes Dis, 2020, 7( 3): 308- 319. DOI: 10.1016/j.gendis.2020.01.014. |
| [4] |
YE Y, SONG YN, HE SF, et al. GINS2 promotes cell proliferation and inhibits cell apoptosis in thyroid cancer by regulating CITED2 and LOXL2[J]. Cancer Gene Ther, 2019, 26( 3-4): 103- 113. DOI: 10.1038/s41417-018-0045-y. |
| [5] |
SEKEDAT MD, FENYÖ D, ROGERS RS, et al. GINS motion reveals replication fork progression is remarkably uniform throughout the yeast genome[J]. Mol Syst Biol, 2010, 6: 353. DOI: 10.1038/msb.2010.8. |
| [6] |
OGINO H, ISHINO S, MAYANAGI K, et al. The GINS complex from the thermophilic archaeon, Thermoplasma acidophilum may function as a homotetramer in DNA replication[J]. Extremophiles, 2011, 15( 4): 529- 539. DOI: 10.1007/s00792-011-0383-2. |
| [7] |
YANG H, LIU XC, ZHU XL, et al. GINS1 promotes the proliferation and migration of glioma cells through USP15-mediated deubiquitination of TOP2A[J]. iScience, 2022, 25( 9): 104952. DOI: 10.1016/j.isci.2022.104952. |
| [8] |
BAXLEY RM, LEUNG W, SCHMIT MM, et al. Bi-allelic MCM10 variants associated with immune dysfunction and cardiomyopathy cause telomere shortening[J]. Nat Commun, 2021, 12( 1): 1626. DOI: 10.1038/s41467-021-21878-x. |
| [9] |
XU XL, CHEN E, MO LH, et al. BRCA1 represses DNA replication initiation through antagonizing estrogen signaling and maintains genome stability in parallel with WEE1-MCM2 signaling during pregnancy[J]. Hum Mol Genet, 2019, 28( 5): 842- 857. DOI: 10.1093/hmg/ddy398. |
| [10] |
AHMAD M, HAMEED Y, KHAN M, et al. Up-regulation of GINS1 highlighted a good diagnostic and prognostic potential of survival in three different subtypes of human cancer[J]. Braz J Biol, 2021, 84: e250575. DOI: 10.1590/1519-6984.250575. |
| [11] |
FU QQ, ZHENG H, WANG X, et al. GINS1 promotes the initiation and progression of bladder cancer by activating the AKT/mTOR/c-Myc signaling pathway[J]. Exp Cell Res, 2024, 440( 1): 114125. DOI: 10.1016/j.yexcr.2024.114125. |
| [12] |
WANG KC, WANG MD, YANG T. Key points in AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma(2023)[J]. J Clin Hepatol, 2023, 39( 9): 2081- 2086. DOI: 10.3969/j.issn.1001-5256.2023.09.008. |
| [13] |
LI Z, ZHU JY. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707. 李照, 朱继业.《原发性肝癌诊疗指南(2024年版)》解读[J]. 临床肝胆病杂志, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707. |
| [14] |
UENO M, ITOH M, KONG LY, et al. PSF1 is essential for early embryogenesis in mice[J]. Mol Cell Biol, 2005, 25( 23): 10528- 10532. DOI: 10.1128/MCB.25.23.10528-10532.2005. |
| [15] |
NAGAHAMA Y, UENO M, MIYAMOTO S, et al. PSF1, a DNA replication factor expressed widely in stem and progenitor cells, drives tumorigenic and metastatic properties[J]. Cancer Res, 2010, 70( 3): 1215- 1224. DOI: 10.1158/0008-5472.CAN-09-3662. |
| [16] |
MAI WH, CHEN CX, LIU QY, et al. Regulation of Notch signaling pathway in immune responses during infection[J]. Chin J Immunol, 2024, 40( 4): 872- 879.
麦文豪, 陈楚溪, 刘巧媛, 等. Notch信号通路在感染过程中对免疫应答的调控作用[J]. 中国免疫学杂志, 2024, 40( 4): 872- 879.
|
| [17] |
ZHOU BH, LIN WL, LONG YL, et al. Notch signaling pathway: Architecture, disease, and therapeutics[J]. Signal Transduct Target Ther, 2022, 7( 1): 95. DOI: 10.1038/s41392-022-00934-y. |
| [18] |
ZHANG XY, SU TH, WU YF, et al. N6-methyladenosine reader YTHDF1 promotes stemness and therapeutic resistance in hepatocellular carcinoma by enhancing NOTCH1 expression[J]. Cancer Res, 2024, 84( 6): 827- 840. DOI: 10.1158/0008-5472.CAN-23-1916. |
| [19] |
WU WR, SHI XD, ZHANG FP, et al. Activation of the Notch1-c-myc-VCAM1 signalling axis initiates liver progenitor cell-driven hepatocarcinogenesis and pulmonary metastasis[J]. Oncogene, 2022, 41( 16): 2340- 2356. DOI: 10.1038/s41388-022-02246-5. |
| [20] |
GRAMANTIERI L, GIOVANNINI C, LANZI A, et al. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma[J]. Liver Int, 2007, 27( 7): 997- 1007. DOI: 10.1111/j.1478-3231.2007.01544.x. |
| [21] |
HU L, XUE F, SHAO MH, et al. Aberrant expression of Notch3 predicts poor survival for hepatocellular carcinomas[J]. Biosci Trends, 2013, 7( 3): 152- 156.
|
| [22] |
GIOVANNINI C, BAGLIONI M, BARON TOALDO M, et al. Notch3 inhibition enhances sorafenib cytotoxic efficacy by promoting GSK3b phosphorylation and p21 down-regulation in hepatocellular carcinoma[J]. Oncotarget, 2013, 4( 10): 1618- 1631. DOI: 10.18632/oncotarget.1221. |
| [23] |
GIOVANNINI C, SALZANO AM, BAGLIONI M, et al. Brivanib in combination with Notch3 silencing shows potent activity in tumour models[J]. Br J Cancer, 2019, 120( 6): 601- 611. DOI: 10.1038/s41416-018-0375-4. |
| [24] |
ZHU WH, LIANG Q, YANG X, et al. Combination of sorafenib and Valproic acid synergistically induces cell apoptosis and inhibits hepatocellular carcinoma growth via down-regulating Notch3 and pAkt[J]. Am J Cancer Res, 2017, 7( 12): 2503- 2514.
|
| [25] |
XIN P, XU XY, DENG CJ, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases[J]. Int Immunopharmacol, 2020, 80: 106210. DOI: 10.1016/j.intimp.2020.106210. |
| [26] |
YI L, LI WD, WANG Y, et al. Effects of aloperine on proliferation, apoptosis and immune escape of colorectal cancer cells by regulating IL-6/JAK1/STAT3 signaling pathway[J]. Chin J Immunol, 2024, 40( 7): 1436- 1440.
伊亮, 李伟东, 王有, 等. 苦豆碱调节IL-6/JAK1/STAT3信号通路对结直肠癌细胞增殖、凋亡和免疫逃逸的影响[J]. 中国免疫学杂志, 2024, 40( 7): 1436- 1440.
|
| [27] |
CARAGLIA M, VITALE G, MARRA M, et al. Alpha-interferon and its effects on signalling pathways within cells[J]. Curr Protein Pept Sci, 2004, 5( 6): 475- 485. DOI: 10.2174/1389203043379378. |
| [28] |
ZHAO ZW, SONG JJ, TANG BF, et al. CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway[J]. J Exp Clin Cancer Res, 2020, 39( 1): 259. DOI: 10.1186/s13046-020-01769-7. |







 DownLoad:
DownLoad: