| [1] |
CHUNG YY, RAHIM MN, HENEGHAN MA. Autoimmune hepatitis and pregnancy: Considerations for the clinician[J]. Expert Rev Clin Immunol, 2022, 18( 4): 325- 333. DOI: 10.1080/1744666X.2022.2044307. |
| [2] |
SMOLKA V, TKACHYK O, EHRMANN J, et al. Acute onset of autoimmune hepatitis in children and adolescents[J]. Hepatobiliary Pancreat Dis Int, 2020, 19( 1): 17- 21. DOI: 10.1016/j.hbpd.2019.08.004. |
| [3] |
SHARMA R, VERNA EC, SÖDERLING J, et al. Increased mortality risk in autoimmune hepatitis: A nationwide population-based cohort study with histopathology[J]. Clin Gastroenterol Hepatol, 2021, 19( 12): 2636- 2647. DOI: 10.1016/j.cgh.2020.10.006. |
| [4] |
TRIVEDI PJ, HIRSCHFIELD GM. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases[J]. Gut, 2021, 70( 10): 1989- 2003. DOI: 10.1136/gutjnl-2020-322362. |
| [5] |
SIRBE C, SIMU GL, SZABO I, et al. Pathogenesis of autoimmune hepatitis-cellular and molecular mechanisms[J]. Int J Mol Sci, 2021, 22( 24): 13578. DOI: 10.3390/ijms222413578. |
| [6] |
WEI YR, LI YM, YAN L, et al. Alterations of gut microbiome in autoimmune hepatitis[J]. Gut, 2020, 69( 3): 569- 577. DOI: 10.1136/gutjnl-2018-317836. |
| [7] |
DYSON JK, DE MARTIN E, DALEKOS GN, et al. Review article: Unanswered clinical and research questions in autoimmune hepatitis-conclusions of the International Autoimmune Hepatitis Group Research Workshop[J]. Aliment Pharmacol Ther, 2019, 49( 5): 528- 536. DOI: 10.1111/apt.15111. |
| [8] |
BRINKMANN V, REICHARD U, GOOSMANN C, et al. Neutrophil extracellular traps kill bacteria[J]. Science, 2004, 303( 5663): 1532- 1535. DOI: 10.1126/science.1092385. |
| [9] |
CASTANHEIRA FVS, KUBES P. Neutrophils and NET in modulating acute and chronic inflammation[J]. Blood, 2019, 133( 20): 2178- 2185. DOI: 10.1182/blood-2018-11-844530. |
| [10] |
MASUDA S, NAKAZAWA D, SHIDA H, et al. NETosis markers: Quest for specific, objective, and quantitative markers[J]. Clin Chim Acta, 2016, 459: 89- 93. DOI: 10.1016/j.cca.2016.05.029. |
| [11] |
TANAKA K, KOIKE Y, SHIMURA T, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model[J]. PLoS One, 2014, 9( 11): e111888. DOI: 10.1371/journal.pone.0111888. |
| [12] |
|
| [13] |
LOOD C, BLANCO LP, PURMALEK MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease[J]. Nat Med, 2016, 22( 2): 146- 153. DOI: 10.1038/nm.4027. |
| [14] |
HAN F, DING ZF, SHI XL, et al. Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice[J]. Redox Biol, 2023, 64: 102787. DOI: 10.1016/j.redox.2023.102787. |
| [15] |
KESHARI RS, VERMA A, BARTHWAL MK, et al. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NET release from human neutrophils[J]. J Cell Biochem, 2013, 114( 3): 532- 540. DOI: 10.1002/jcb.24391. |
| [16] |
ZHAO XH, YANG L, CHANG N, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling[J]. Cell Death Dis, 2020, 11( 5): 379. DOI: 10.1038/s41419-020-2582-1. |
| [17] |
RADWAN FFY, HOSSAIN A, GOD JM, et al. Reduction of myeloid-derived suppressor cells and lymphoma growth by a natural triterpenoid[J]. J Cell Biochem, 2015, 116( 1): 102- 114. DOI: 10.1002/jcb.24946. |
| [18] |
CHI BJ, WANG SQ, BI S, et al. Effects of ganoderic acid A on lipopolysaccharide-induced proinflammatory cytokine release from primary mouse microglia cultures[J]. Exp Ther Med, 2018, 15( 1): 847- 853. DOI: 10.3892/etm.2017.5472. |
| [19] |
CHENG Y, XIE P. Ganoderic acid A holds promising cytotoxicity on human glioblastoma mediated by incurring apoptosis and autophagy and inactivating PI3K/AKT signaling pathway[J]. J Biochem Mol Toxicol, 2019, 33( 11): e22392. DOI: 10.1002/jbt.22392. |
| [20] |
XU LX, YAN LJ, HUANG SP. Ganoderic acid A against cyclophosphamide-induced hepatic toxicity in mice[J]. J Biochem Mol Toxicol, 2019, 33( 4): e22271. DOI: 10.1002/jbt.22271. |
| [21] |
MA JQ, ZHANG YJ, TIAN ZK. Anti-oxidant, anti-inflammatory and anti-fibrosis effects of ganoderic acid A on carbon tetrachloride induced nephrotoxicity by regulating the Trx/TrxR and JAK/ROCK pathway[J]. Chem Biol Interact, 2021, 344: 109529. DOI: 10.1016/j.cbi.2021.109529. |
| [22] |
TADOKORO T, MORISHITA A, SAKAMOTO T, et al. Galectin-9 ameliorates fulminant liver injury[J]. Mol Med Rep, 2017, 16( 1): 36- 42. DOI: 10.3892/mmr.2017.6606. |
| [23] |
RANI R, TANDON A, WANG J, et al. Stellate cells orchestrate concanavalin A-induced acute liver damage[J]. Am J Pathol, 2017, 187( 9): 2008- 2019. DOI: 10.1016/j.ajpath.2017.05.015. |
| [24] |
WANG KC, WU WR, JIANG XW, et al. Multi-omics analysis reveals the protection of gasdermin D in concanavalin A-induced autoimmune hepatitis[J]. Microbiol Spectr, 2022, 10( 5): e0171722. DOI: 10.1128/spectrum.01717-22. |
| [25] |
XU HL, LIU LL, CHEN YX, et al. The chemical character of polysaccharides from processed Morindae officinalis and their effects on anti-liver damage[J]. Int J Biol Macromol, 2019, 141: 410- 421. DOI: 10.1016/j.ijbiomac.2019.08.213. |
| [26] |
HE GW, GÜNTHER C, KREMER AE, et al. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury[J]. Gut, 2017, 66( 4): 716- 723. DOI: 10.1136/gutjnl-2015-311247. |
| [27] |
LI XH, ZHANG YT, WANG JP, et al. zVAD alleviates experimental autoimmune hepatitis in mice by increasing the sensitivity of macrophage to TNFR1-dependent necroptosis[J]. J Autoimmun, 2022, 133: 102904. DOI: 10.1016/j.jaut.2022.102904. |
| [28] |
LIU FL, SHI KJ, DONG JJ, et al. Ganoderic acid A attenuates high-fat-diet-induced liver injury in rats by regulating the lipid oxidation and liver inflammation[J]. Arch Pharm Res, 2020, 43( 7): 744- 754. DOI: 10.1007/s12272-020-01256-9. |
| [29] |
SAFFARZADEH M, JUENEMANN C, QUEISSER MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones[J]. PLoS One, 2012, 7( 2): e32366. DOI: 10.1371/journal.pone.0032366. |
| [30] |
REN AH, DONG YB, MIAO RZ, et al. Effect of ganoderic acid A on glycolysis and its key rate-limiting enzymes of non-small cell lung cancer PC9 cells[J]. J Jilin Univ(Med Ed), 2024, 50( 3): 682- 688. DOI: 10.13481/j.1671-587X.20240312. |
| [31] |
LV XC, WU Q, CAO YJ, et al. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition[J]. Food Funct, 2022, 13( 10): 5820- 5837. DOI: 10.1039/d1fo03219d. |
| [32] |
ZHU J, DING JX, LI SY, et al. Ganoderic acid A ameliorates non-alcoholic streatohepatitis(NASH) induced by high-fat high-cholesterol diet in mice[J]. Exp Ther Med, 2022, 23( 4): 308. DOI: 10.3892/etm.2022.11237. |
| [33] |
MA F, CHANG XJ, WANG GY, et al. Streptococcus suis serotype 2 stimulates neutrophil extracellular traps formation via activation of p38 MAPK and ERK1/2[J]. Front Immunol, 2018, 9: 2854. DOI: 10.3389/fimmu.2018.02854. |
| [34] |
MIELI-VERGANI G, VERGANI D, CZAJA AJ, et al. Autoimmune hepatitis[J]. Nat Rev Dis Primers, 2018, 4: 18017. DOI: 10.1038/nrdp.2018.17. |



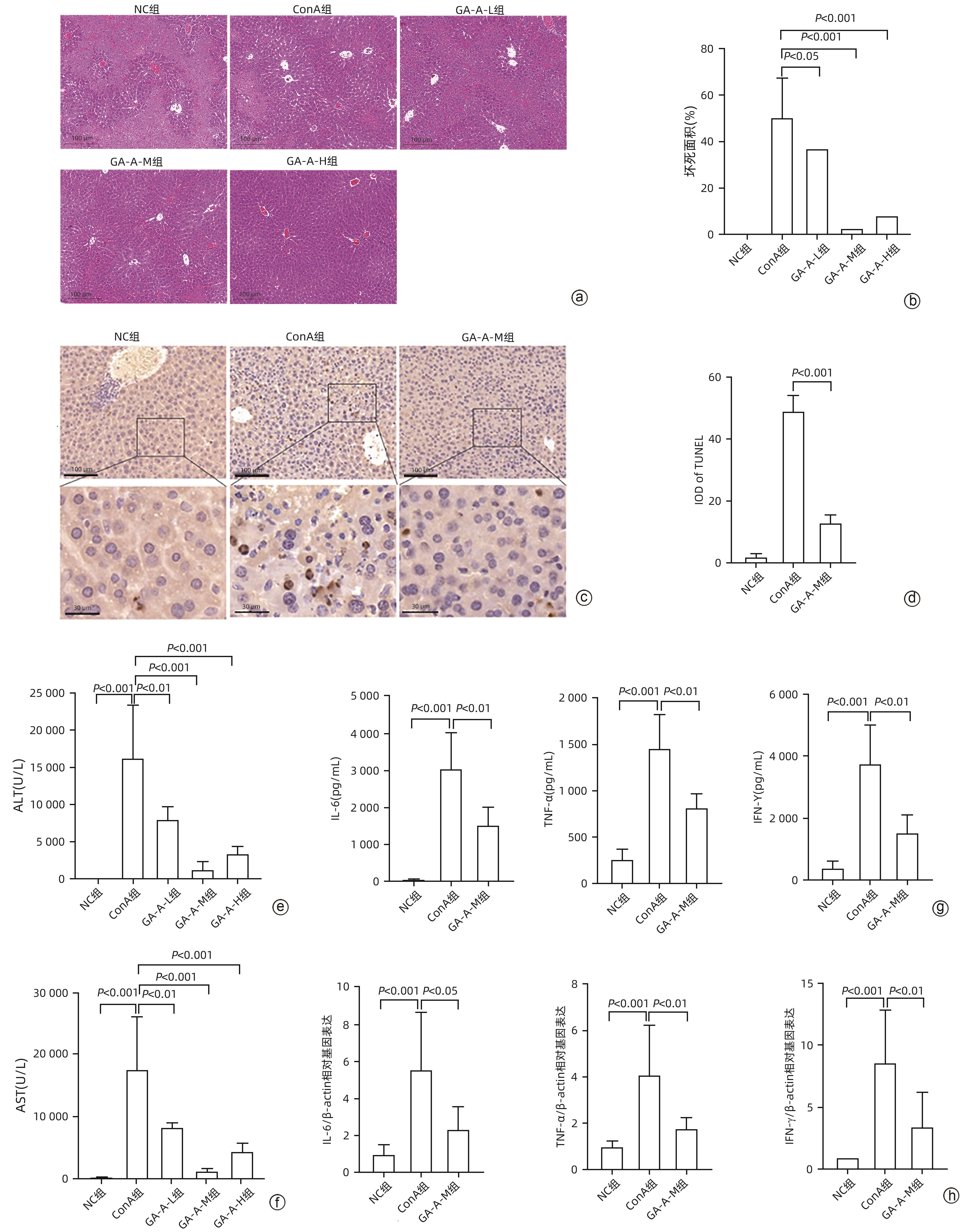




 DownLoad:
DownLoad: