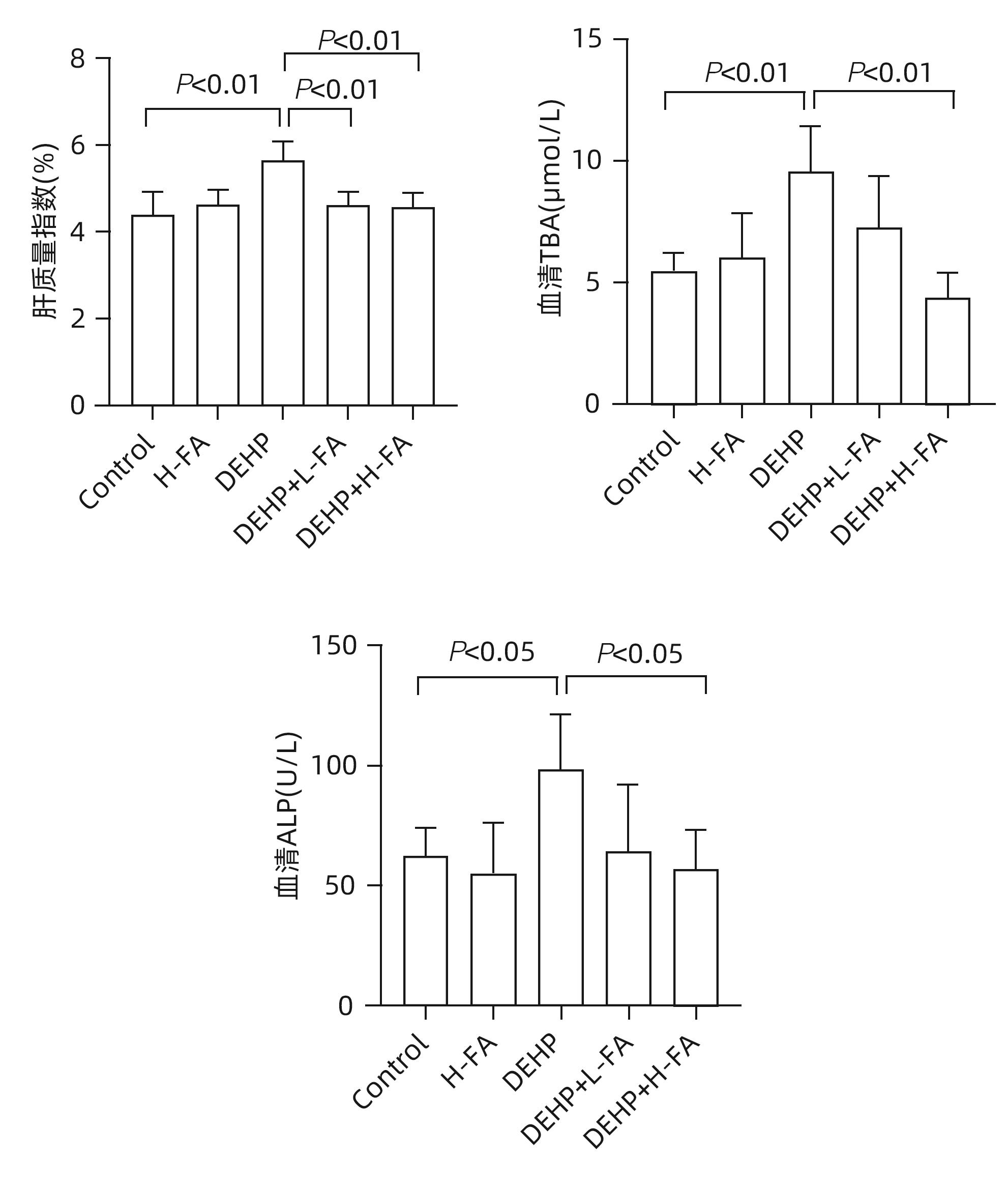
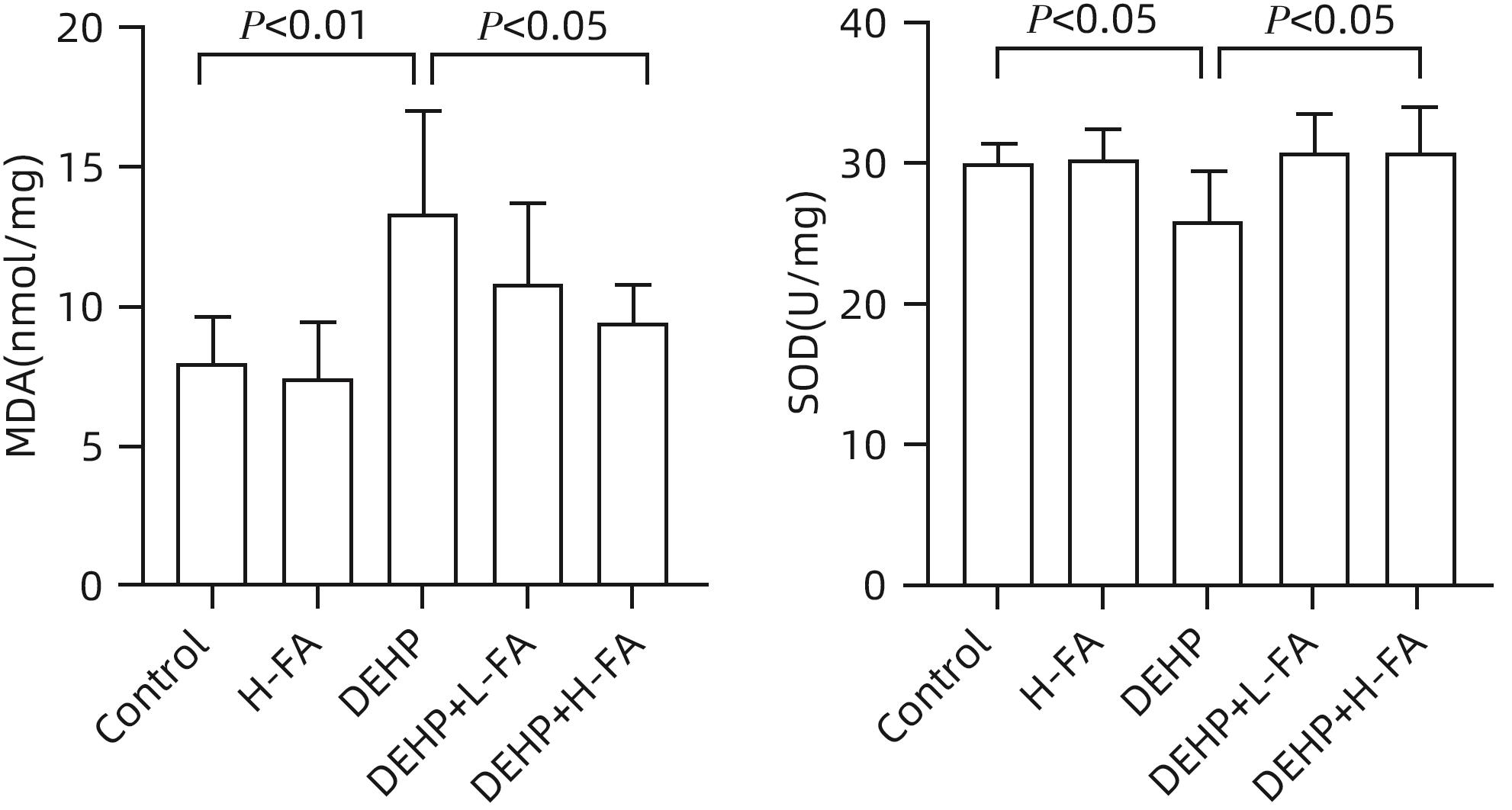
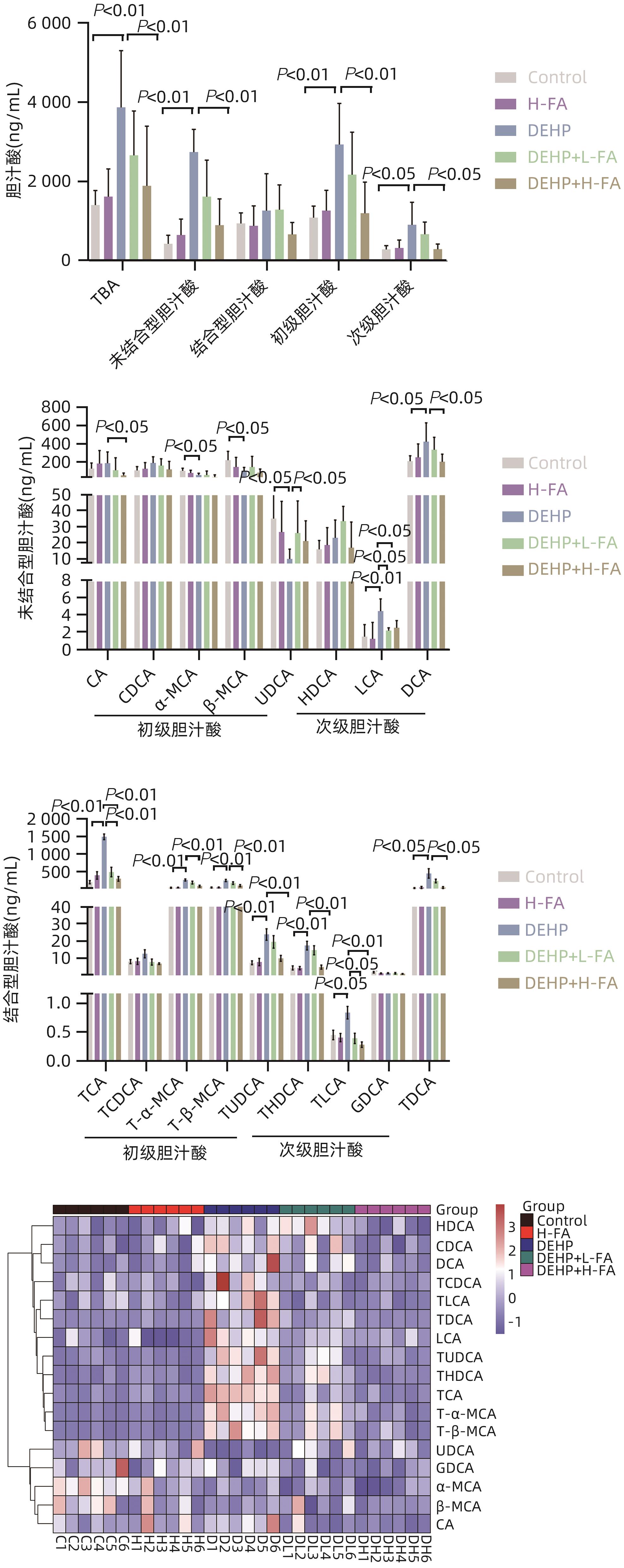
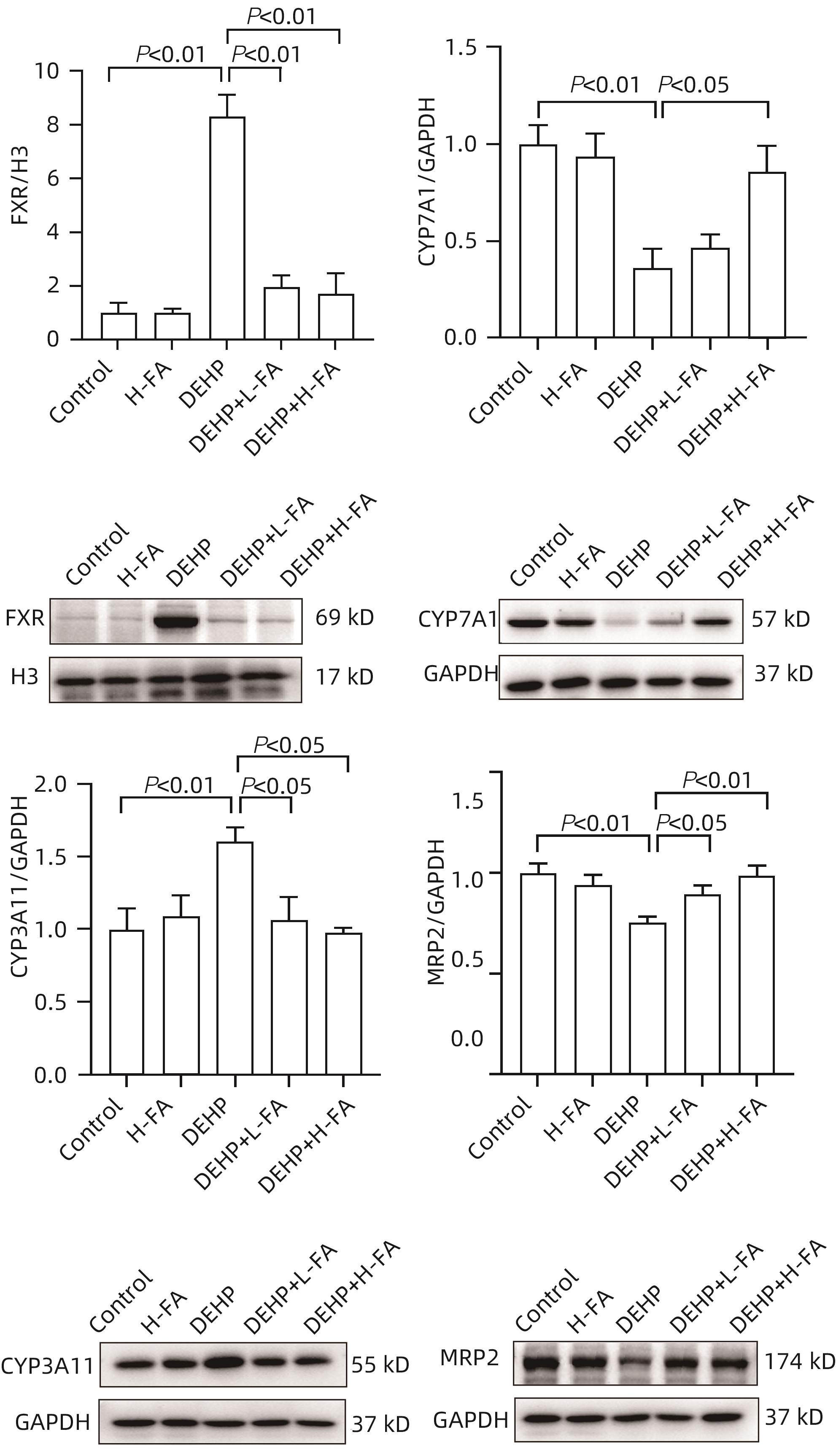
| [1] |
BAGEL S, DESSAIGNE B, BOURDEAUX D, et al. Influence of lipid type on bis(2-ethylhexyl)phthalate(DEHP) leaching from infusion line sets in parenteral nutrition[J]. JPEN J Parenter Enteral Nutr, 2011, 35( 6): 770- 775. DOI: 10.1177/0148607111414021. |
| [2] |
ZHAO F, ZHANG L, QU MC, et al. Obeticholic acid alleviates intrauterine growth restriction induced by di-ethyl-hexyl phthalate in pregnant female mice by improving bile acid disorder[J]. Environ Sci Pollut Res Int, 2023, 30( 51): 110956- 110969. DOI: 10.1007/s11356-023-30149-9. |
| [3] |
GALLEGO-LOPEZ MDC, OJEDA ML, ROMERO-HERRERA I, et al. Folic acid homeostasis and its pathways related to hepatic oxidation in adolescent rats exposed to binge drinking[J]. Antioxidants(Basel), 2022, 11( 2): 362. DOI: 10.3390/antiox11020362. |
| [4] |
ZHANG HQ, ZUO YW, ZHAO HC, et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis[J]. Front Nutr, 2022, 9: 989311. DOI: 10.3389/fnut.2022.989311. |
| [5] |
CHEN S, YANG MY, WANG R, et al. Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota[J]. Eur J Nutr, 2022, 61( 4): 2015- 2031. DOI: 10.1007/s00394-021-02769-9. |
| [6] |
JIANG L, GAI XC, NI Y, et al. Folic acid protects against tuberculosis-drug-induced liver injury in rats and its potential mechanism by metabolomics[J]. J Nutr Biochem, 2023, 112: 109214. DOI: 10.1016/j.jnutbio.2022.109214. |
| [7] |
ZHANG YJ, GUO JL, XUE JC, et al. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment[J]. Environ Pollut, 2021, 291: 118106. DOI: 10.1016/j.envpol.2021.118106. |
| [8] |
GAITANTZI H, HAKENBERG P, THEOBALD J, et al. Di(2-ethylhexyl) phthalate and its role in developing cholestasis: An in vitro study on different liver cell types[J]. J Pediatr Gastroenterol Nutr, 2018, 66( 2): e28- e35. DOI: 10.1097/MPG.0000000000001813. |
| [9] |
WEI XJ, YANG DQ, ZHANG BY, et al. Di-(2-ethylhexyl) phthalate increases plasma glucose and induces lipid metabolic disorders via FoxO1 in adult mice[J]. Sci Total Environ, 2022, 842: 156815. DOI: 10.1016/j.scitotenv.2022.156815. |
| [10] |
ZHOU YH, ZHOU YZ, LI YF, et al. Targeted bile acid profiles reveal the liver injury amelioration of Da-Chai-Hu Decoction against ANIT- and BDL-induced cholestasis[J]. Front Pharmacol, 2022, 13: 959074. DOI: 10.3389/fphar.2022.959074. |
| [11] |
WANG GF, LI YY, SHI R, et al. Yinchenzhufu decoction protects against alpha-naphthylisothiocyanate-induced acute cholestatic liver injury in mice by ameliorating disordered bile acid homeostasis and inhibiting inflammatory responses[J]. J Ethnopharmacol, 2020, 254: 112672. DOI: 10.1016/j.jep.2020.112672. |
| [12] |
LE YB, WANG KH, ZOU L. Mechanism of taurocholic acid in promoting the progression of liver cirrhosis[J]. J Clin Hepatol, 2021, 37( 11): 2658- 2662. DOI: 10.3969/j.issn.1001-5256.2021.11.037. |
| [13] |
LI CZ, HUANG XW, ZHANG ZP, et al. Research progress in role of gut-liver axis in occurrence and development of atherosclerosis[J]. J Jilin Univ Med Ed, 2023, 49( 6): 1669- 1676. DOI: 10.13481/j.1671-587X.20230636. |
| [14] |
TRAUNER M, FUCHS CD. Novel therapeutic targets for cholestatic and fatty liver disease[J]. Gut, 2022, 71( 1): 194- 209. DOI: 10.1136/gutjnl-2021-324305. |
| [15] |
HAN X, LIN C, LIU H, et al. Allocholic acid protects against α- naphthylisothiocyanate-induced cholestasis in mice by ameliorating disordered bile acid homeostasis[J]. J Appl Toxicol, 2024, 44( 4): 582- 594. DOI: 10.1002/jat.4562. |
| [16] |
CHAI J, CAI SY, LIU XC, et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis[J]. J Hepatol, 2015, 63( 6): 1440- 1448. DOI: 10.1016/j.jhep.2015.07.016. |
| [17] |
PAULUSMA CC, KOTHE MJ, BAKKER CT, et al. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver[J]. Hepatology, 2000, 31( 3): 684- 693. DOI: 10.1002/hep.510310319. |
| [18] |
ZU Y, LIU YN, LAN LL, et al. Consecutive baicalin treatment relieves its accumulation in rats with intrahepatic cholestasis by increasing MRP2 expression[J]. Heliyon, 2023, 9( 1): e12689. DOI: 10.1016/j.heliyon.2022.e12689. |
| [19] |
RAZORI MV, MAIDAGAN PM, CIRIACI N, et al. Anticholestatic mechanisms of ursodeoxycholic acid in lipopolysaccharide-induced cholestasis[J]. Biochem Pharmacol, 2019, 168: 48- 56. DOI: 10.1016/j.bcp.2019.06.009. |
| [20] |
HINOSHITA E, TAGUCHI K, INOKUCHI A, et al. Decreased expression of an ATP-binding cassette transporter, MRP2, in human livers with hepatitis C virus infection[J]. J Hepatol, 2001, 35( 6): 765- 773. DOI: 10.1016/s0168-8278(01)00216-1. |







 DownLoad:
DownLoad: