| [1] |
COOKE GS, FLOWER B, CUNNINGHAM E, et al. Progress towards elimination of viral hepatitis: A Lancet Gastroenterology& Hepatology Commission update[J]. Lancet Gastroenterol Hepatol, 2024, 9( 4): 346- 365. DOI: 10.1016/S2468-1253(23)00321-7. |
| [2] |
TOY M, HUTTON D, JIA JD, et al. Costs and health impact of delayed implementation of a national hepatitis B treatment program in China[J]. J Glob Health, 2022, 12: 04043. DOI: 10.7189/jogh.12.04043. |
| [3] |
PÉNEAU C, IMBEAUD S, BELLA TL, et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma[J]. Gut, 2022, 71( 3): 616- 626. DOI: 10.1136/gutjnl-2020-323153. |
| [4] |
ERKEN R, LOUKACHOV V, van DORT K, et al. Quantified integrated hepatitis B virus is related to viral activity in patients with chronic hepatitis B[J]. Hepatology, 2022, 76( 1): 196- 206. DOI: 10.1002/hep.32352. |
| [5] |
DONG Z, LI JR, ZHAO ZX, et al. Molecular epidemiology of hepatitis B virus genotypes and subgenotypes in ethnic minority populations, Yunnan Province, China[J]. Epidemiol Infect, 2021, 150: e11. DOI: 10.1017/S0950268821002326. |
| [6] |
SORIANO V, ALVAREZ C, EDAGWA B, et al. Ultra-long-acting(XLA) antivirals for chronic viral hepatitis[J]. Int J Infect Dis, 2022, 114: 45- 50. DOI: 10.1016/j.ijid.2021.10.052. |
| [7] |
KAKALOU C, POLYCHRONIDOU E, DROSOU V, et al. RiskRadar: Development and pilot results of a technical intervention targeting combination prevention regarding HIV, viral hepatitis, sexually transmitted infections and tuberculosis[J]. BMC Infect Dis, 2021, 21( Suppl 2): 866. DOI: 10.1186/s12879-021-06501-0. |
| [8] |
CUI FQ, SHEN LP, LI L, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China[J]. Emerg Infect Dis, 2017, 23( 5): 765- 772. DOI: 10.3201/eid2305.161477. |
| [9] |
LIU J, WANG XY, WANG Q, et al. Hepatitis B virus infection among 90 million pregnant women in 2853 Chinese Counties, 2015-2020: A national observational study[J]. Lancet Reg Health West Pac, 2021, 16: 100267. DOI: 10.1016/j.lanwpc.2021.100267. |
| [10] |
HU M, CHEN W. Assessment of total economic burden of chronic hepatitis B(CHB)-related diseases in Beijing and Guangzhou, China[J]. Value Health, 2009, 12( Suppl 3): S89- S92. DOI: 10.1111/j.1524-4733.2009.00636.x. |
| [11] |
PENG CY, CHIEN RN, LIAW YF. Hepatitis B virus-related decompensated liver cirrhosis: Benefits of antiviral therapy[J]. J Hepatol, 2012, 57( 2): 442- 450. DOI: 10.1016/j.jhep.2012.02.033. |
| [12] |
FATTOVICH G, BORTOLOTTI F, DONATO F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors[J]. J Hepatol, 2008, 48( 2): 335- 352. DOI: 10.1016/j.jhep.2007.11.011. |
| [13] |
ABARA WE, QASEEM A, SCHILLIE S, et al. Hepatitis B vaccination, screening, and linkage to care: Best practice advice from the American college of physicians and the centers for disease control and prevention[J]. Ann Intern Med, 2017, 167( 11): 794- 804. DOI: 10.7326/M17-1106. |
| [14] |
TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. |
| [15] |
TOY M, HUTTON D, HARRIS AM, et al. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States[J]. Clin Infect Dis, 2022, 74( 2): 210- 217. DOI: 10.1093/cid/ciab405. |
| [16] |
XIAO YZ, HOWELL J, van GEMERT C, et al. Enhancing the hepatitis B care cascade in Australia: A cost-effectiveness model[J]. J Viral Hepat, 2020, 27( 5): 526- 536. DOI: 10.1111/jvh.13252. |
| [17] |
WOLFFRAM I. A comprehensive screening for hepatitis B and C as an effective means of cancer prevention and as a prerequisite for elimination of chronic viral hepatitis-data and comments on a discussion[J]. Dtsch Med Wochenschr, 2023, 148( 4): 175- 182. DOI: 10.1055/a-1972-4118. |
| [18] |
|
| [19] |
CONNERS EE, PANAGIOTAKOPOULOS L, HOFMEISTER MG, et al. Screening and testing for hepatitis B virus infection: CDC recommendations-United States, 2023[J]. MMWR Recomm Rep, 2023, 72( 1): 1- 25. DOI: 10.15585/mmwr.rr7201a1. |
| [20] |
ALLARD NL, MACLACHLAN JH, TRAN L, et al. Time for universal hepatitis B screening for Australian adults[J]. Med J Aust, 2021, 215( 3): 103- 105. DOI: 10.5694/mja2.51114. |
| [21] |
HARRIS AM, OSINUBI A, NELSON NP, et al. The hepatitis B care cascade using administrative claims data, 2016[J]. Am J Manag Care, 2020, 26( 8): 331- 338. DOI: 10.37765/ajmc.2020.44069. |
| [22] |
OGAWA E, YEO YH, DANG N, et al. Diagnosis rates of chronic hepatitis B in privately insured patients in the United States[J]. JAMA Netw Open, 2020, 3( 4): e201844. DOI: 10.1001/jamanetworkopen.2020.1844. |
| [23] |
VIJAYADEVA V, SPRADLING PR, MOORMAN AC, et al. Hepatitis B virus infection testing and prevalence among Asian and Pacific Islanders[J]. Am J Manag Care, 2014, 20( 4): e98- e104.
|
| [24] |
|
| [25] |
CHEUNG KW, LAO TT. Hepatitis B-Vertical transmission and the prevention of mother-to-child transmission[J]. Best Pract Res Clin Obstet Gynaecol, 2020, 68: 78- 88. DOI: 10.1016/j.bpobgyn.2020.02.014. |
| [26] |
|
| [27] |
CUI F, WOODRING J, CHAN P, et al. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China[J]. Int J Epidemiol, 2018, 47( 5): 1529- 1537. DOI: 10.1093/ije/dyy077. |
| [28] |
HAN GR, CAO MK, ZHAO W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection[J]. J Hepatol, 2011, 55( 6): 1215- 1221. DOI: 10.1016/j.jhep.2011.02.032. |
| [29] |
MIN DY, HUANG WY, YANG JY, et al. Familial aggregation of hepatitis B virus infection and its influence factors in minority areas of Guizhou Province[J]. Chin J Public Health, 2016, 32( 2): 183- 187. DOI: 10.11847/zgggws2016-32-02-15. |
| [30] |
YU YK, ZHU X, CHEN ZX, et al. Status and influencing factors of new hepatitis B virus infection in anzhou district, Mianyang city[J]. Acta Acad Med Sin, 2022, 44( 6): 996- 1003. DOI: 10.3881/j.issn.1000-503X.15058. |
| [31] |
YUEN MF. Need to improve awareness and management of hepatitis B reactivation in patients receiving immunosuppressive therapy[J]. Hepatol Int, 2016, 10( 1): 102- 105. DOI: 10.1007/s12072-015-9694-1. |
| [32] |
PÉREZ-ALVAREZ R, DÍAZ-LAGARES C, GARCÍA-HERNÁNDEZ F, et al. Hepatitis B virus(HBV) reactivation in patients receiving tumor necrosis factor(TNF)-targeted therapy: Analysis of 257 cases[J]. Medicine, 2011, 90( 6): 359- 371. DOI: 10.1097/MD.0b013e3182380a76. |
| [33] |
YOO S, LEE DB, SHIM JH, et al. Risk of hepatitis B virus reactivation in patients treated with immunotherapy for anti-cancer treatment[J]. Clin Gastroenterol Hepatol, 2022, 20( 4): 898- 907. DOI: 10.1016/j.cgh.2021.06.019. |
| [34] |
BAO YP, LARNEY S, PEACOCK A, et al. Prevalence of HIV, HCV and HBV infection and sociodemographic characteristics of people who inject drugs in China: A systematic review and meta-analysis[J]. Int J Drug Policy, 2019, 70: 87- 93. DOI: 10.1016/j.drugpo.2019.05.005. |
| [35] |
ZHANG FJ, ZHU H, WU YS, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010-12: A retrospective observational cohort study[J]. Lancet Infect Dis, 2014, 14( 11): 1065- 1072. DOI: 10.1016/S1473-3099(14)70946-6. |
| [36] |
|
| [37] |
XU YL, YANG XS, LIU KY, et al. Prevalence of and associated factors for HIV and HBV infections among men who have sex with men in Beijing, China[J]. J Cap Med Univ, 2014, 35( 1): 96- 100. DOI: 10.3969/j.issn.1006-7795.2014.01.021. |
| [38] |
ZHANG X, ZHU X, JI Y, et al. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus[J]. Biosci Rep, 2019, 39( 3): BSR20181715. DOI: 10.1042/BSR20181715. |
| [39] |
CAMPBELL C, WANG T, MCNAUGHTON AL, et al. Risk factors for the development of hepatocellular carcinoma(HCC) in chronic hepatitis B virus(HBV) infection: A systematic review and meta-analysis[J]. J Viral Hepat, 2021, 28( 3): 493- 507. DOI: 10.1111/jvh.13452. |
| [40] |
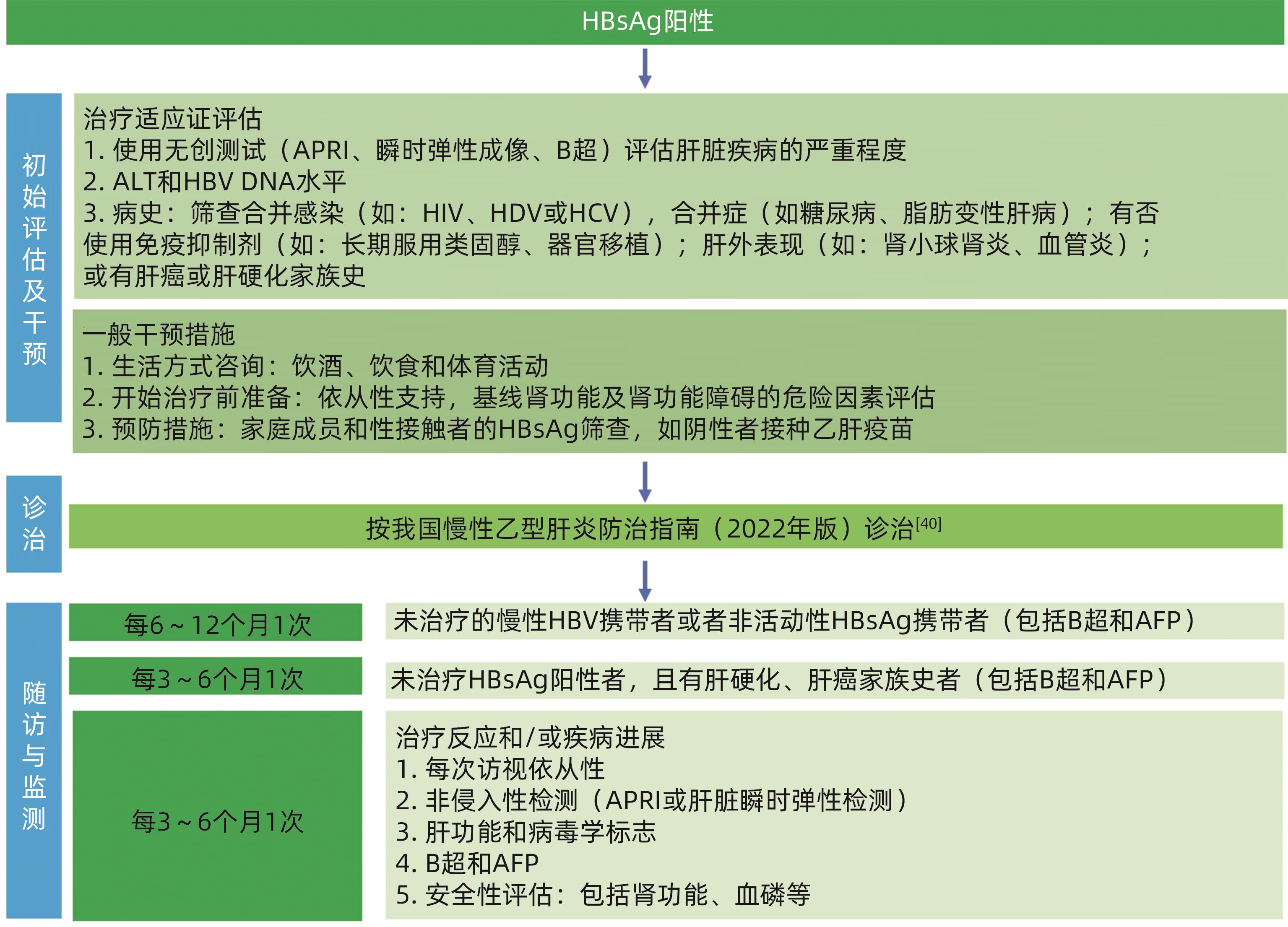
Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. J Pract Hepatol, 2023, 26( 3): S18- S39. DOI: 10.3969/j.issn.1672-5069.2023.03.040. |
| [41] |
LI D, MALLORY T, SATOMURA S. AFP-L3: A new generation of tumor marker for hepatocellular carcinoma[J]. Clin Chim Acta, 2001, 313( 1-2): 15- 19. DOI: 10.1016/s0009-8981(01)00644-1. |
| [42] |
NGUYEN HB, LE XT, NGUYEN HH, et al. Diagnostic value of hTERT mRNA and in combination with AFP, AFP-L3%, des-γ-carboxyprothrombin for screening of hepatocellular carcinoma in liver cirrhosis patients HBV or HCV-related[J]. Cancer Inform, 2022, 21: 11769351221100730. DOI: 10.1177/11769351221100730. |
| [43] |
Society of Prevention and Control of Infectious Diseases, Chinese Preventive Medicine Association. Expert consensus on the role of hematological markers in the early clinical screening of hepatocellular carcinoma[J]. Chin J Viral Dis, 2021, 11( 5): 334- 340. DOI: 10.16505/j.2095-0136.2021.0049. |
| [44] |
LIN XJ, CHONG YT, GUO ZW, et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study[J]. Lancet Oncol, 2015, 16( 7): 804- 815. DOI: 10.1016/S1470-2045(15)00048-0. |
| [45] |
ZHU W, SHI P, LIANG A, et al. The combination of serum oligosaccharide chain(G-test), alpha-fetoprotein, and aspartate aminotransferase to alanine aminotransferase ratio provides the optimal diagnostic value for early detection of hepatocellular carcinoma[J]. BMC Cancer, 2022, 22( 1): 1061. DOI: 10.1186/s12885-022-10139-9. |
| [46] |
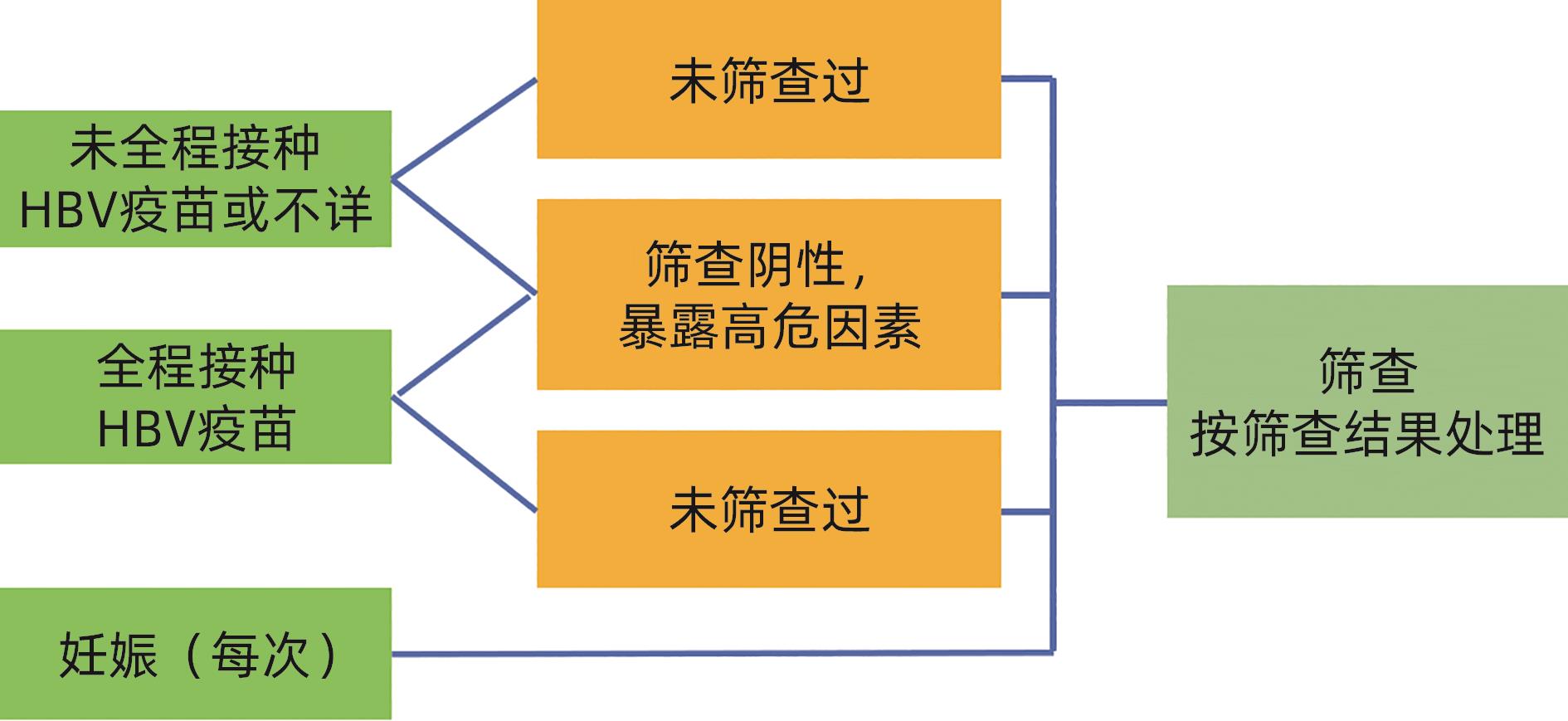
Working Committee of Promoting the Elimination of Viral Hepatitis of Chinese Preventive Medicine Association, Society of Prevention and Control of Infectious Diseases of Chinese Preventive Medicine Association. Expert recommendations on hepatitis B vaccination in adults[J]. Chin J Viral Dis, 2024, 14( 4): 310- 316. DOI: 10.16505/j.2095-0136.2024.0047. |







 DownLoad:
DownLoad: