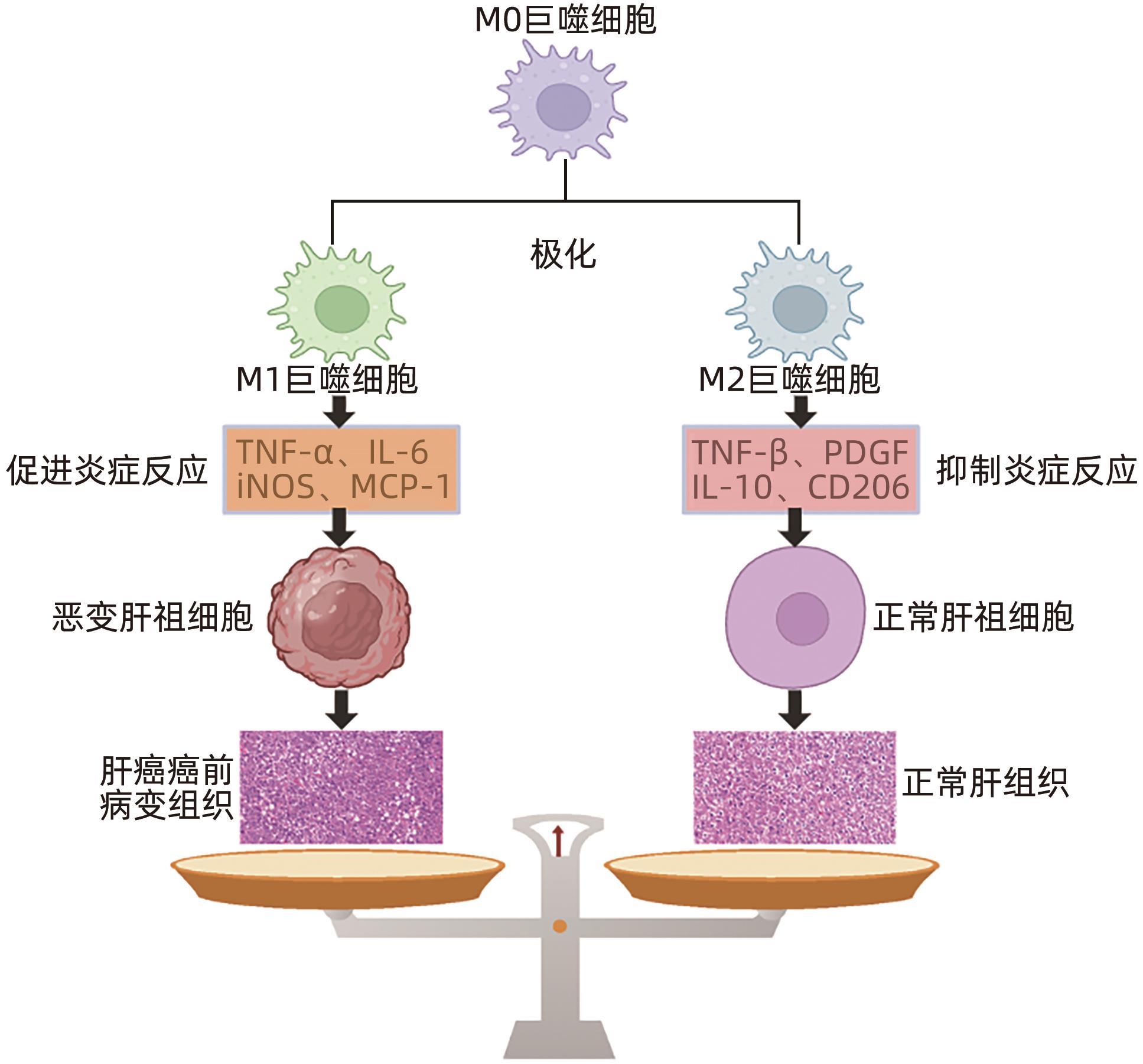
| [1] |
|
| [2] |
WANG C, VEGNA S, JIN HJ, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer[J]. Nature, 2019, 574( 7777): 268- 272. DOI: 10.1038/s41586-019-1607-3. |
| [3] |
RENZULLI M, BISELLI M, BROCCHI S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm[J]. Gut, 2018, 67( 9): 1674- 1682. DOI: 10.1136/gutjnl-2017-315384. |
| [4] |
JIAO JZ, LI JT, YAN SG, et al. Current research status of precancerous dysplastic nodules in hepatocellular carcinoma[J]. J Clin Hepatol, 2017, 33( 5): 974- 978. DOI: 10.3969/j.issn.1001-5256.2017.05.039. |
| [5] |
Professional Committee for Prevention and Control of Hepatobiliary and Pancreatic Diseases of Chinese Preventive Medicine Association; Professional Committee for Hepatology, Chinese Research Hospital Association; Chinese Society of Hepatology, Chinese Medical Association, et al. Guideline for stratified screening and surveillance of primary liver cancer(2020 edition)[J]. J Clin Hepatol, 2021, 37( 2): 286- 295. DOI: 10.3969/j.issn.1001-5256.2021.02.009. |
| [6] |
MARQUARDT JU, ANDERSEN JB, THORGEIRSSON SS. Functional and genetic deconstruction of the cellular origin in liver cancer[J]. Nat Rev Cancer, 2015, 15( 11): 653- 667. DOI: 10.1038/nrc4017. |
| [7] |
ZHU LQ, FINKELSTEIN D, GAO CL, et al. Multi-organ mapping of cancer risk[J]. Cell, 2016, 166( 5): 1132- 1146. DOI: 10.1016/j.cell.2016.07.045. |
| [8] |
MIYAJIMA A, TANAKA M, ITOH T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming[J]. Cell Stem Cell, 2014, 14( 5): 561- 574. DOI: 10.1016/j.stem.2014.04.010. |
| [9] |
BRIA A, MARDA J, ZHOU JM, et al. Hepatic progenitor cell activation in liver repair[J]. Liver Res, 2017, 1( 2): 81- 87. DOI: 10.1016/j.livres.2017.08.002. |
| [10] |
WU CC, LIN CJ, KUO KK, et al. Correlation between cancer stem cells, inflammation and malignant transformation in a DEN-induced model of hepatic carcinogenesis[J]. Curr Issues Mol Biol, 2022, 44( 7): 2879- 2886. DOI: 10.3390/cimb44070198. |
| [11] |
PU WJ, ZHU H, ZHANG MJ, et al. Bipotent transitional liver progenitor cells contribute to liver regeneration[J]. Nat Genet, 2023, 55( 4): 651- 664. DOI: 10.1038/s41588-023-01335-9. |
| [12] |
LIU WT, GAO L, HOU XJ, et al. TWEAK signaling-induced ID1 expression drives malignant transformation of hepatic progenitor cells during hepatocarcinogenesis[J]. Adv Sci, 2023, 10( 18): e2300350. DOI: 10.1002/advs.202300350. |
| [13] |
NIO K, YAMASHITA T, KANEKO S. The evolving concept of liver cancer stem cells[J]. Mol Cancer, 2017, 16( 1): 4. DOI: 10.1186/s12943-016-0572-9. |
| [14] |
THAN NN, NEWSOME PN. Stem cells for liver regeneration[J]. QJM, 2014, 107( 6): 417- 421. DOI: 10.1093/qjmed/hcu013. |
| [15] |
YAN ZJ, CHEN L, WANG HY. To be or not to be: The double-edged sword roles of liver progenitor cells[J]. Biochim Biophys Acta Rev Cancer, 2023, 1878( 3): 188870. DOI: 10.1016/j.bbcan.2023.188870. |
| [16] |
SIA D, VILLANUEVA A, FRIEDMAN SL, et al. Liver cancer cell of origin, molecular class, and effects onPatient prognosis[J]. Gastroenterology, 2017, 152( 4): 745- 761. DOI: 10.1053/j.gastro.2016.11.048. |
| [17] |
HAIDERI SS, MCKINNON AC, TAYLOR AH, et al. Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury[J]. NPJ Regen Med, 2017, 2: 14. DOI: 10.1038/s41536-017-0017-0. |
| [18] |
ZENG JX, JING YY, WU QL, et al. Autophagy is required for hepatic differentiation of hepatic progenitor cells via Wnt signaling pathway[J]. Biomed Res Int, 2021, 2021: 6627506. DOI: 10.1155/2021/6627506. |
| [19] |
NATI M, CHUNG KJ, CHAVAKIS T. The role of innate immune cells in nonalcoholic fatty liver disease[J]. J Innate Immun, 2022, 14( 1): 31- 41. DOI: 10.1159/000518407. |
| [20] |
KHURANA A, NAVIK U, ALLAWADHI P, et al. Spotlight on liver macrophages for halting liver disease progression and injury[J]. Expert Opin Ther Targets, 2022, 26( 8): 707- 719. DOI: 10.1080/14728222.2022.2133699. |
| [21] |
SOUCIE EL, WENG ZM, GEIRSDÓTTIR L, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells[J]. Science, 2016, 351( 6274): aad5510. DOI: 10.1126/science.aad5510. |
| [22] |
BLÉRIOT C, DUPUIS T, JOUVION G, et al. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection[J]. Immunity, 2015, 42( 1): 145- 158. DOI: 10.1016/j.immuni.2014.12.020. |
| [23] |
|
| [24] |
LUO Y, XIAO JH. Inflammatory auxo-action in the stem cell division theory of cancer[J]. PeerJ, 2023, 11: e15444. DOI: 10.7717/peerj.15444. |
| [25] |
LI XF, CHEN C, XIANG DM, et al. Chronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significance[J]. Hepatology, 2017, 66( 6): 1934- 1951. DOI: 10.1002/hep.29372. |
| [26] |
SICA A, INVERNIZZI P, MANTOVANI A. Macrophage plasticity and polarization in liver homeostasis and pathology[J]. Hepatology, 2014, 59( 5): 2034- 2042. DOI: 10.1002/hep.26754. |
| [27] |
MURRAY PJ, ALLEN JE, BISWAS SK, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines[J]. Immunity, 2014, 41( 1): 14- 20. DOI: 10.1016/j.immuni.2014.06.008. |
| [28] |
SHAPOURI-MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease[J]. J Cell Physiol, 2018, 233( 9): 6425- 6440. DOI: 10.1002/jcp.26429. |
| [29] |
JIN K, LI T, SÁNCHEZ-DUFFHUES G, et al. Involvement of inflammation and its related microRNAs in hepatocellular carcinoma[J]. Oncotarget, 2017, 8( 13): 22145- 22165. DOI: 10.18632/oncotarget.13530. |
| [30] |
YANG L, ZHANG Y. Tumor-associated macrophages: From basic research to clinical application[J]. J Hematol Oncol, 2017, 10( 1): 58. DOI: 10.1186/s13045-017-0430-2. |
| [31] |
MAO YL, WANG BK, XU X, et al. Glycyrrhizic acid promotes M1 macrophage polarization in murine bone marrow-derived macrophages associated with the activation of JNK and NF-κB[J]. Mediators Inflamm, 2015, 2015: 372931. DOI: 10.1155/2015/372931. |
| [32] |
TANG Y, KITISIN K, JOGUNOORI W, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling[J]. Proc Natl Acad Sci U S A, 2008, 105( 7): 2445- 2450. DOI: 10.1073/pnas.0705395105. |
| [33] |
HAN CY, YANG Y, SHENG YJ, et al. The mechanism of lncRNA-CRNDE in regulating tumour-associated macrophage M2 polarization and promoting tumour angiogenesis[J]. J Cellular Molecular Medi, 2021, 25( 9): 4235- 4247. DOI: 10.1111/jcmm.16477. |
| [34] |
GUNASSEKARAN GR, POONGKAVITHAI VADEVOO SM, BAEK MC, et al. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages[J]. Biomaterials, 2021, 278: 121137. DOI: 10.1016/j.biomaterials.2021.121137. |
| [35] |
ORECCHIONI M, GHOSHEH Y, PRAMOD AB, et al. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages[J]. Front Immunol, 2019, 10: 1084. DOI: 10.3389/fimmu.2019.01084. |
| [36] |
RINGELHAN M, PFISTER D, O’CONNOR T, et al. The immunology of hepatocellular carcinoma[J]. Nat Immunol, 2018, 19( 3): 222- 232. DOI: 10.1038/s41590-018-0044-z. |
| [37] |
Bureau of Medical Administration, National Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China(2019 edition)[J]. J Clin Hepatol, 2020, 36( 2): 277- 292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [38] |
CHEN JM, CHEN L, ZERN MA, et al. The diversity and plasticity of adult hepatic progenitor cells and their niche[J]. Liver Int, 2017, 37( 9): 1260- 1271. DOI: 10.1111/liv.13377. |
| [39] |
HOU XJ, YE F, LI XY, et al. Immune response involved in liver damage and the activation of hepatic progenitor cells during liver tumorigenesis[J]. Cell Immunol, 2018, 326: 52- 59. DOI: 10.1016/j.cellimm.2017.08.004. |
| [40] |
KAUR S, SIDDIQUI H, BHAT MH. Hepatic progenitor cells in action: Liver regeneration or fibrosis?[J]. Am J Pathol, 2015, 185( 9): 2342- 2350. DOI: 10.1016/j.ajpath.2015.06.004. |
| [41] |
ALEKSANDROVA K, BOEING H, NÖTHLINGS U, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer[J]. Hepatology, 2014, 60( 3): 858- 871. DOI: 10.1002/hep.27016. |
| [42] |
YANG X, SHAO CC, DUAN LX, et al. Oncostatin M promotes hepatic progenitor cell activation and hepatocarcinogenesis via macrophage-derived tumor necrosis factor-Α[J]. Cancer Lett, 2021, 517: 46- 54. DOI: 10.1016/j.canlet.2021.05.039. |
| [43] |
LI L, CUI L, LIN P, et al. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers[J]. Cell Stem Cell, 2023, 30( 3): 283- 299. e 9. DOI: 10.1016/j.stem.2023.01.009. |
| [44] |
GALDIERO MR, BONAVITA E, BARAJON I, et al. Tumor associated macrophages and neutrophils in cancer[J]. Immunobiology, 2013, 218( 11): 1402- 1410. DOI: 10.1016/j.imbio.2013.06.003. |
| [45] |
GOSWAMI KK, GHOSH T, GHOSH S, et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment[J]. Cell Immunol, 2017, 316: 1- 10. DOI: 10.1016/j.cellimm.2017.04.005. |
| [46] |
van HUL N, LANTHIER N, ESPAÑOL SUÑER R, et al. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury[J]. Am J Pathol, 2011, 179( 4): 1839- 1850. DOI: 10.1016/j.ajpath.2011.06.042. |



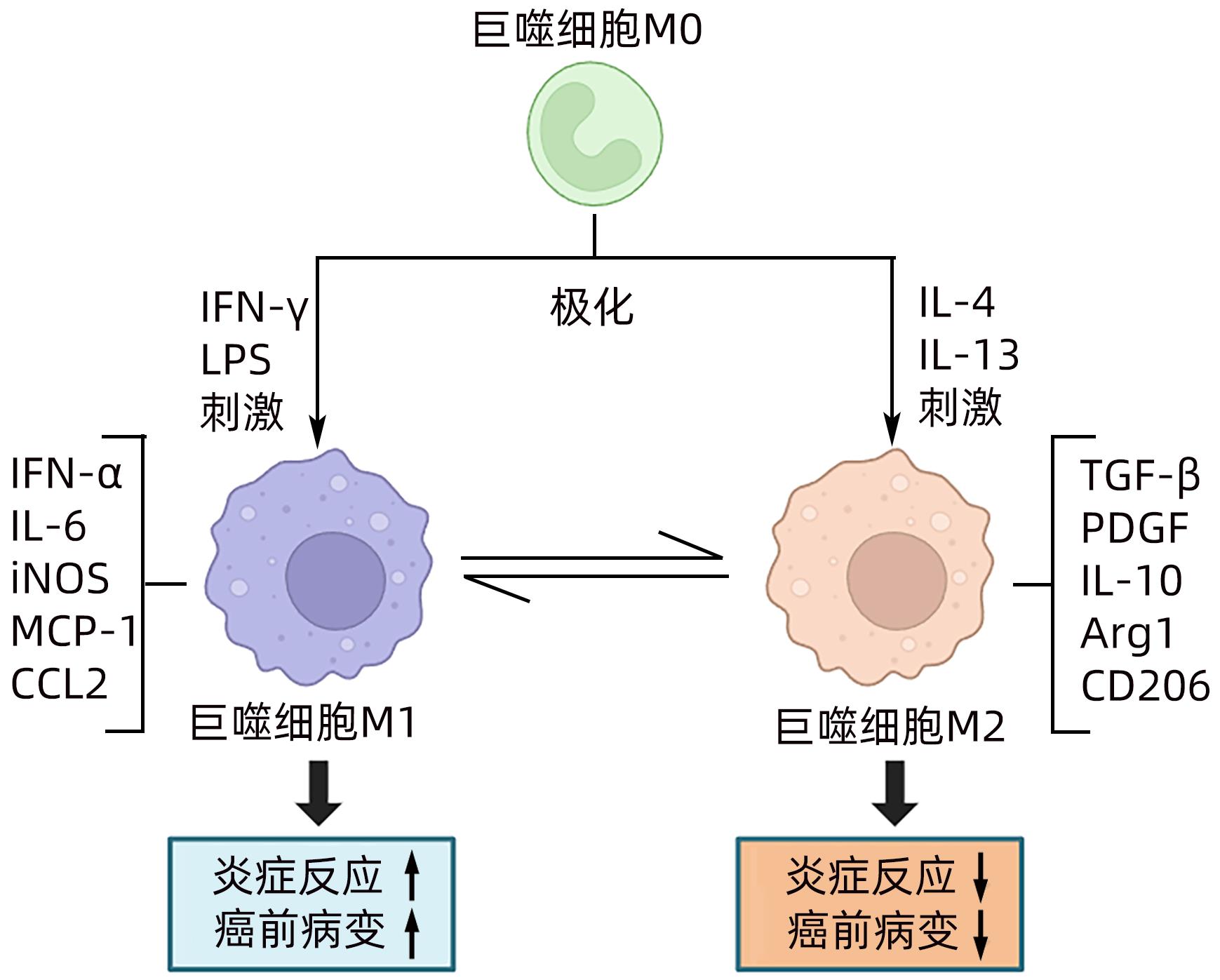




 DownLoad:
DownLoad: