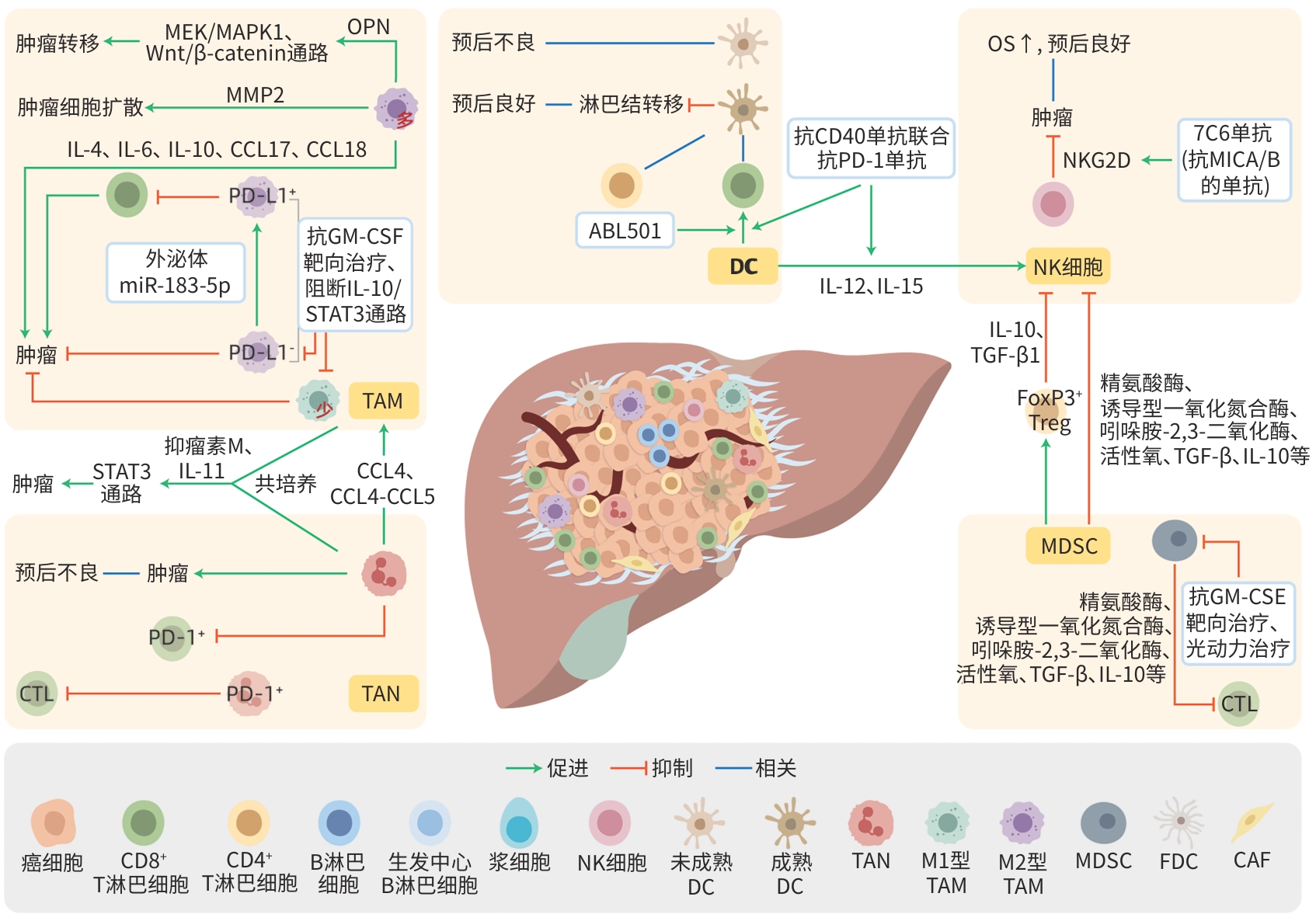
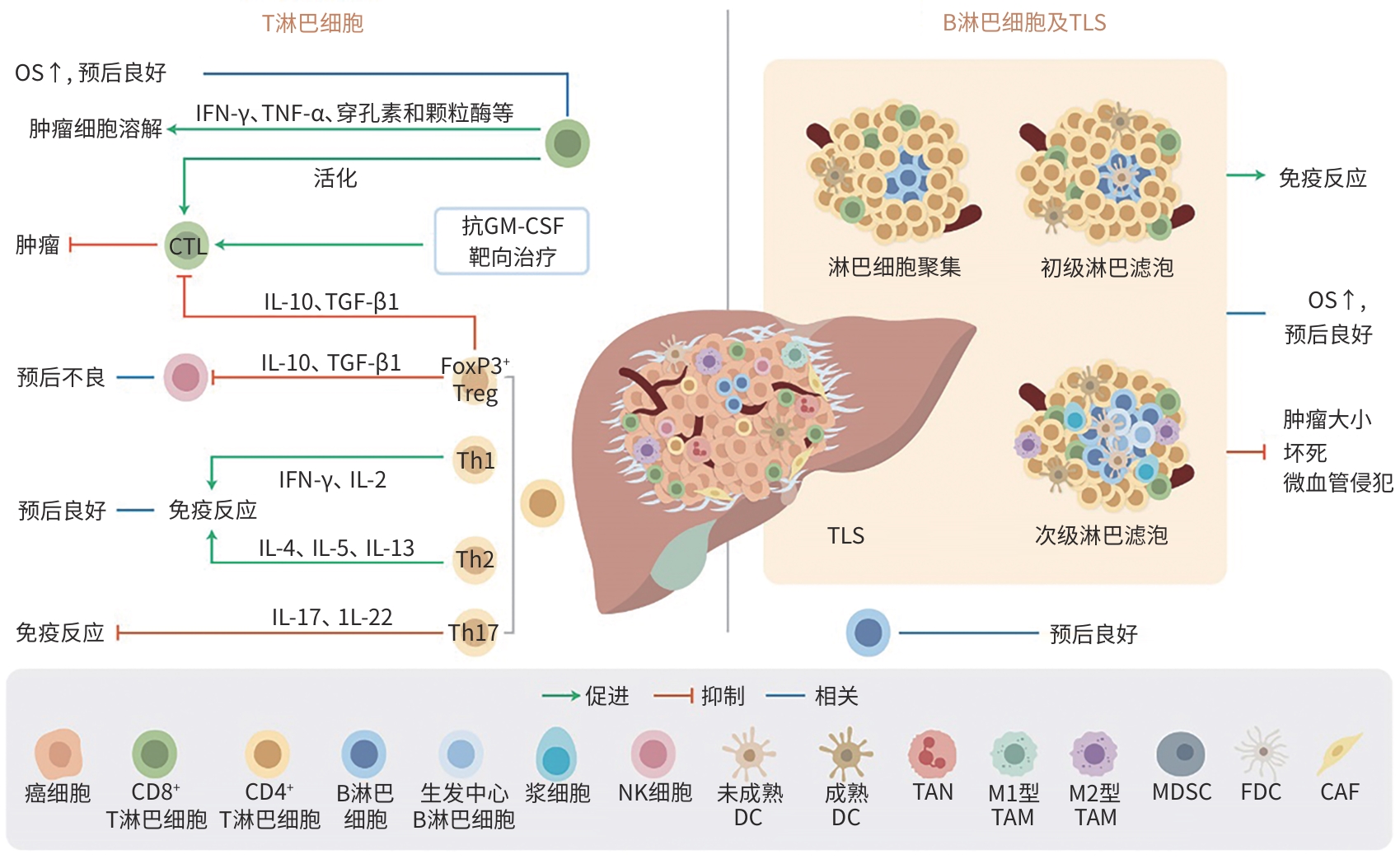
肝内胆管癌间质-免疫微环境内成分及其相互作用
DOI: 10.12449/JCH250331
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:张秋露负责查找文献、撰写论文;李卓负责论文修改;郭丽梅、刘从容负责拟定写作思路,指导文章撰写、论文修改并最后定稿。
Components of tumor stroma-immune microenvironment and their interactions in intrahepatic cholangiocarcinoma
-
摘要: 肝内胆管癌(ICC)是一种高度恶性的肝脏肿瘤,由于早期无明显症状并缺乏有效的治疗手段,患者5年生存率极低。肿瘤间质-免疫微环境(TSIME)是肿瘤发生与演进过程中局部所处的一个动态变化的复杂生态系统,由多种细胞成分和非细胞成分共同组成,在ICC的发生、增殖、侵袭与进展过程中起着重要的作用,在一定程度上也决定了ICC的异质性及恶性程度。本文就ICC-TSIME内的T淋巴细胞、B淋巴细胞、自然杀伤细胞、树突状细胞、中性粒细胞、巨噬细胞、髓源性抑制细胞等多种细胞成分,趋化因子、细胞因子等非细胞成分,以及其与肿瘤细胞间复杂多变的相互作用机制进行综述,同时对免疫细胞与肿瘤细胞的空间相互作用关系进行综述,以期为ICC的免疫治疗提供潜在的研究方向,为未来更高效、更精确的ICC治疗带来新思路。Abstract: Intrahepatic cholangiocarcinoma (ICC) is a highly malignant liver tumor, and due to the absence of symptoms in its early stage and the lack of effective treatment measures, patients tend to have an extremely low 5-year survival rate. The tumor stroma-immune microenvironment (TSIME) is a complex ecosystem that changes dynamically during tumorigenesis and evolution and consists of a variety of cellular and non-cellular components, and it plays an important role in the development, proliferation, invasion, and progression of ICC and determines the heterogeneity and malignancy of ICC to a certain degree. This article reviews the cellular components (such as T cells, B cells, natural killer cells, dendritic cells, neutrophils, macrophages, myeloid-derived suppressor cells) and non-cellular components (such as chemokines and cytokines) within the ICC TSIME, as well as the complex mechanisms of interaction between these components, and it also reviews the spatial interactions between immune cells and tumor cells, in order to provide potential research directions for ICC immunotherapy and new ideas for the effective and precise treatment of ICC in the future.
-
Key words:
- Cholangiocarcinoma /
- Tumor Microenvironment /
- Lymphocytes
-
-
[1] BRINDLEY PJ, BACHINI M, ILYAS SI, et al. Cholangiocarcinoma[J]. Nat Rev Dis Primers, 2021, 7( 1): 65. DOI: 10.1038/s41572-021-00300-2. [2] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [3] Expert Committee of Expert Consensus on Pathological Diagnosis of Intrahepatic Cholangiocarcinoma( 2022 version). Expert consensus on pathological diagnosis of intrahepatic cholangiocarcinoma(2022 version)[J]. Chin J Pathol, 2022, 51( 9): 819- 827. DOI: 10.3760/cma.j.cn112151-20220517-00423.《肝内胆管癌病理诊断专家共识(2022版)》编写专家委员会. 肝内胆管癌病理诊断专家共识(2022版)[J]. 中华病理学杂志, 2022, 51( 9): 819- 827. DOI: 10.3760/cma.j.cn112151-20220517-00423. [4] WANG GY, HE CH. Progress in liver transplantation for intrahepatic cholangiocarcinoma[J]. Organ Transplantation, 2023, 14( 6): 789- 796. DOI: 10.3969/j.issn.1674-7445.2023144.汪国营, 何才华. 肝移植治疗肝内胆管癌的研究进展[J]. 器官移植, 2023, 14( 6): 789- 796. DOI: 10.3969/j.issn.1674-7445.2023144. [5] MORIS D, PALTA M, KIM C, et al. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians[J]. CA Cancer J Clin, 2023, 73( 2): 198- 222. DOI: 10.3322/caac.21759. [6] KOSHIOL J, YU BB, KABADI SM, et al. Epidemiologic patterns of biliary tract cancer in the United States: 2001-2015[J]. BMC Cancer, 2022, 22( 1): 1178. DOI: 10.1186/s12885-022-10286-z. [7] JIN MZ, JIN WL. The updated landscape of tumor microenvironment and drug repurposing[J]. Signal Transduct Target Ther, 2020, 5( 1): 166. DOI: 10.1038/s41392-020-00280-x. [8] FABRIS L, PERUGORRIA MJ, MERTENS J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma[J]. Liver Int, 2019, 39( Suppl 1): 63- 78. DOI: 10.1111/liv.14098. [9] GENTILINI A, PASTORE M, MARRA F, et al. The role of stroma in cholangiocarcinoma: The intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium[J]. Int J Mol Sci, 2018, 19( 10): 2885. DOI: 10.3390/ijms19102885. [10] LOEUILLARD E, CONBOY CB, GORES GJ, et al. Immunobiology of cholangiocarcinoma[J]. JHEP Rep, 2019, 1( 4): 297- 311. DOI: 10.1016/j.jhepr.2019.06.003. [11] LIN J, DAI YT, SANG C, et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities[J]. J Immunother Cancer, 2022, 10( 7): e004892. DOI: 10.1136/jitc-2022-004892. [12] RUFFOLO LI, JACKSON KM, KUHLERS PC, et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity[J]. Gut, 2022, 71( 7): 1386- 1398. DOI: 10.1136/gutjnl-2021-324109. [13] LIU D, HEIJ LR, CZIGANY Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma[J]. J Exp Clin Cancer Res, 2022, 41( 1): 127. DOI: 10.1186/s13046-022-02340-2. [14] XIA T, LI KY, NIU N, et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses[J]. J Hematol Oncol, 2022, 15( 1): 37. DOI: 10.1186/s13045-022-01253-z. [15] FABRIS L, SATO K, ALPINI G, et al. The tumor microenvironment in cholangiocarcinoma progression[J]. Hepatology, 2021, 73( Suppl 1): 75- 85. DOI: 10.1002/hep.31410. [16] CHEN Z, YU MC, YAN JL, et al. PNOC expressed by B cells in cholangiocarcinoma was survival related and LAIR2 could be a T cell exhaustion biomarker in tumor microenvironment: Characterization of immune microenvironment combining single-cell and bulk sequencing technology[J]. Front Immunol, 2021, 12: 647209. DOI: 10.3389/fimmu.2021.647209. [17] THEPMALEE C, PANYA A, JUNKING M, et al. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells[J]. Hum Vaccin Immunother, 2018, 14( 6): 1423- 1431. DOI: 10.1080/21645515.2018.1431598. [18] SAUTÈS-FRIDMAN C, PETITPREZ F, CALDERARO J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy[J]. Nat Rev Cancer, 2019, 19( 6): 307- 325. DOI: 10.1038/s41568-019-0144-6. [19] DOI N, INO Y, FUSE M, et al. Correlation of vein-rich tumor microenvironment of intrahepatic cholangiocarcinoma with tertiary lymphoid structures and patient outcome[J]. Mod Pathol, 2024, 37( 2): 100401. DOI: 10.1016/j.modpat.2023.100401. [20] DING GY, MA JQ, YUN JP, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma[J]. J Hepatol, 2022, 76( 3): 608- 618. DOI: 10.1016/j.jhep.2021.10.030. [21] SHI HN, LI ZS, ZHU MC. Circulating immune cells predict prognosis and clinical response to chemotherapy in cholangiocarcinoma[J]. Curr Med Chem, 2024. DOI: 10.2174/0109298673296618240424095548.[ Online ahead of print] [22] OLIVIERO B, VARCHETTA S, MELE D, et al. MICA/B-targeted antibody promotes NK cell-driven tumor immunity in patients with intrahepatic cholangiocarcinoma[J]. Oncoimmunology, 2022, 11( 1): 2035919. DOI: 10.1080/2162402X.2022.2035919. [23] VITA F, OLAIZOLA I, AMATO F, et al. Heterogeneity of cholangiocarcinoma immune biology[J]. Cells, 2023, 12( 6): 846. DOI: 10.3390/cells12060846. [24] DIGGS LP, RUF B, MA C, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma[J]. J Hepatol, 2021, 74( 5): 1145- 1154. DOI: 10.1016/j.jhep.2020.11.037. [25] CAZZETTA V, FRANZESE S, CARENZA C, et al. Natural killer-dendritic cell interactions in liver cancer: Implications for immunotherapy[J]. Cancers(Basel), 2021, 13( 9): 2184. DOI: 10.3390/cancers13092184. [26] SUNG E, KO M, WON JY, et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation[J]. Mol Ther, 2022, 30( 8): 2800- 2816. DOI: 10.1016/j.ymthe.2022.05.003. [27] WANG WM, GAO Y, XU JJ, et al. A NRF2 regulated and the immunosuppressive microenvironment reversed nanoplatform for cholangiocarcinoma photodynamic-gas therapy[J]. Adv Sci(Weinh), 2024, 11( 14): e2307143. DOI: 10.1002/advs.202307143. [28] XUE RD, ZHANG QM, CAO Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity[J]. Nature, 2022, 612( 7938): 141- 147. DOI: 10.1038/s41586-022-05400-x. [29] ZHOU ZJ, WANG PC, SUN RQ, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3[J]. J Immunother Cancer, 2021, 9( 3): e001946. DOI: 10.1136/jitc-2020-001946. [30] WATANABE A, HARIMOTO N, ARAKI K, et al. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma[J]. Anticancer Res, 2019, 39( 2): 941- 947. DOI: 10.21873/anticanres.13197. [31] ZHOU MH, WANG CQ, LU SN, et al. Tumor-associated macrophages in cholangiocarcinoma: Complex interplay and potential therapeutic target[J]. EBioMedicine, 2021, 67: 103375. DOI: 10.1016/j.ebiom.2021.103375. [32] ZENG JH, LIU ZK, SUN SW, et al. Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells[J]. Oncol Lett, 2018, 15( 6): 8681- 8686. DOI: 10.3892/ol.2018.8372. [33] SUN DL, LUO TC, DONG PP, et al. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma[J]. J Cell Biochem, 2020, 121( 4): 2828- 2838. DOI: 10.1002/jcb.29514. [34] CHENG K, CAI N, ZHU JH, et al. Tumor-associated macrophages in liver cancer: From mechanisms to therapy[J]. Cancer Commun(Lond), 2022, 42( 11): 1112- 1140. DOI: 10.1002/cac2.12345. [35] RAGGI C, CORRENTI M, SICA A, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages[J]. J Hepatol, 2017, 66( 1): 102- 115. DOI: 10.1016/j.jhep.2016.08.012. [36] QUARANTA V, BALLARÒ C, GIANNELLI G. Macrophages orchestrate the liver tumor microenvironment[J]. Cancers(Basel), 2024, 16( 9): 1772. DOI: 10.3390/cancers16091772. [37] LUO CB, XIN HY, ZHOU ZJ, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression[J]. Hepatology, 2022, 76( 4): 982- 999. DOI: 10.1002/hep.32387. [38] TOMLINSON JL, VALLE JW, ILYAS SI. Immunobiology of cholangiocarcinoma[J]. J Hepatol, 2023, 79( 3): 867- 875. DOI: 10.1016/j.jhep.2023.05.010. [39] LIN YL, CAI Q, CHEN Y, et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase[J]. Hepatology, 2022, 75( 1): 28- 42. DOI: 10.1002/hep.32099. [40] AFFO S, YU LX, SCHWABE RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer[J]. Annu Rev Pathol, 2017, 12: 153- 186. DOI: 10.1146/annurev-pathol-052016-100322. [41] ZHENG Y, ZHOU C, YU XX, et al. Osteopontin promotes metastasis of intrahepatic cholangiocarcinoma through recruiting MAPK1 and mediating Ser675 phosphorylation of β-Catenin[J]. Cell Death Dis, 2018, 9( 2): 179. DOI: 10.1038/s41419-017-0226-x. [42] GRACIA-SANCHO J, CAPARRÓS E, FERNÁNDEZ-IGLESIAS A, et al. Role of liver sinusoidal endothelial cells in liver diseases[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 6): 411- 431. DOI: 10.1038/s41575-020-00411-3. [43] COGLIATI B, YASHASWINI CN, WANG S, et al. Friend or foe? The elusive role of hepatic stellate cells in liver cancer[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 10): 647- 661. DOI: 10.1038/s41575-023-00821-z. [44] YUAN H, LIN ZL, LIU YJ, et al. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness[J]. Cancer Cell Int, 2020, 20( 1): 586. DOI: 10.1186/s12935-020-01687-w. [45] ZHU CB, MA JQ, ZHU K, et al. Spatial immunophenotypes predict clinical outcome in intrahepatic cholangiocarcinoma[J]. JHEP Rep, 2023, 5( 8): 100762. DOI: 10.1016/j.jhepr.2023.100762. [46] LIN YP, PENG LH, DONG LQ, et al. Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma[J]. Cancer Discov, 2022, 12( 10): 2350- 2371. DOI: 10.1158/2159-8290.CD-21-1640. [47] HONG JH, YONG CH, HENG H, et al. Integrative multiomics enhancer activity profiling identifies therapeutic vulnerabilities in cholangiocarcinoma of different etiologies[J]. Gut, 2024, 73( 6): 966- 984. DOI: 10.1136/gutjnl-2023-330483. [48] CHEN JL, TANG Y, QIN DL, et al. ALOX5 acts as a key role in regulating the immune microenvironment in intrahepatic cholangiocarcinoma, recruiting tumor-associated macrophages through PI3K pathway[J]. J Transl Med, 2023, 21( 1): 923. DOI: 10.1186/s12967-023-04804-1. [49] XU LX, ZHANG Y, LIN ZL, et al. FASN-mediated fatty acid biosynthesis remodels immune environment in Clonorchis sinensis infection-related intrahepatic cholangiocarcinoma[J]. J Hepatol, 2024, 81( 2): 265- 277. DOI: 10.1016/j.jhep.2024.03.016. -



 PDF下载 ( 1621 KB)
PDF下载 ( 1621 KB)

 下载:
下载: