胆-肠轴在肝内胆管癌发生发展中的作用机制
DOI: 10.12449/JCH250330
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:刘艳负责课题设计,拟定写作思路,指导及撰写文章;俞雪负责分析资料及撰写文章;沈天皓、周诚、刘雨负责收集文献,分析资料;李炜、蒋霆辉、朱永强负责指导写作思路并最后定稿。
Mechanism of action of bile-gut axis in the development and progression of intrahepatic cholangiocarcinoma
-
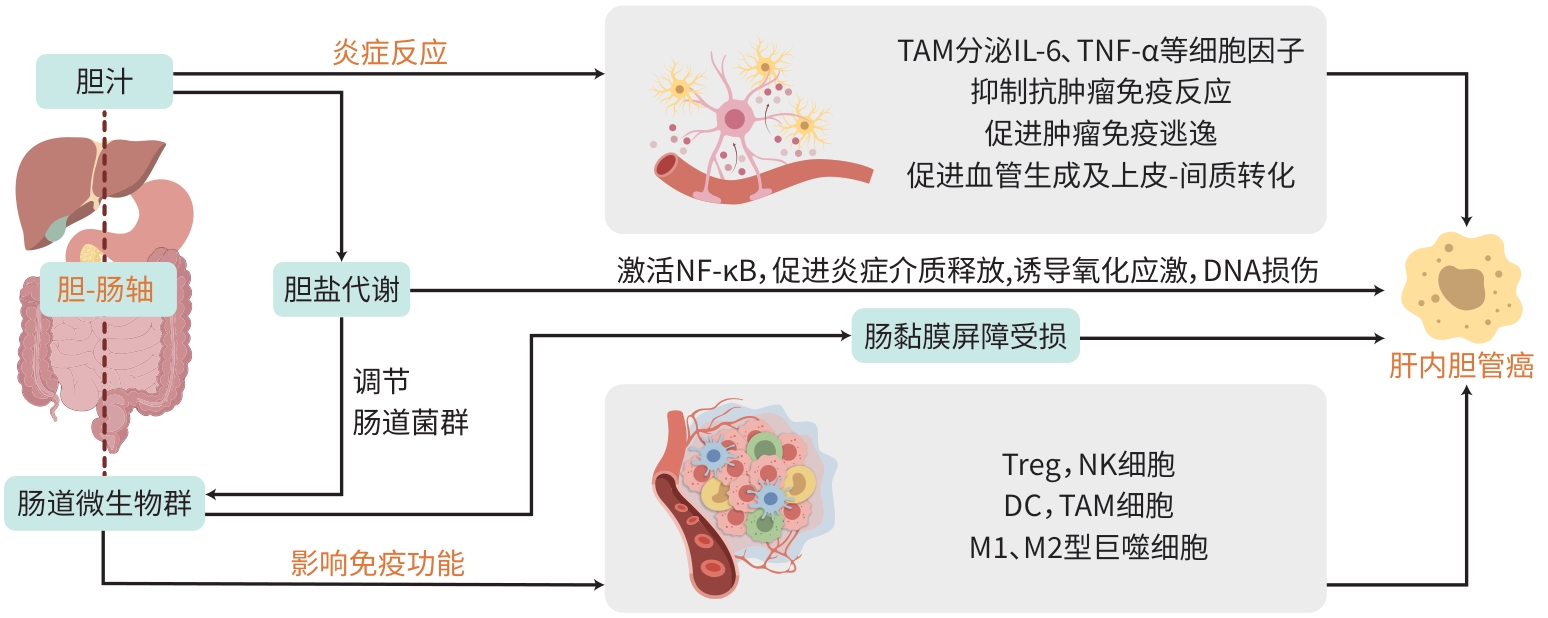
摘要: 肝内胆管癌是一种预后极差的恶性肿瘤,其发病机制复杂且尚未完全明确。近年来,越来越多的研究聚焦于胆-肠轴在肝内胆管癌发生发展中的作用。胆-肠轴是指胆汁和肠道微生物群之间的复杂交互作用,包括胆盐代谢、微生物群的动态变化、炎症反应以及免疫系统的调节等多方面内容。本文系统阐述了胆-肠轴在肝内胆管癌中的潜在机制,特别是微生物群失调、胆盐代谢异常、慢性炎症反应及免疫系统交互作用等方面,旨在为未来研究提供新的视角和可能的治疗靶点,推动肝内胆管癌的早期诊断和有效治疗。Abstract: Intrahepatic cholangiocarcinoma is a malignant tumor with an extremely poor prognosis, and its pathogenesis is complex and remains unclear. In recent years, more and more studies have focused on the role of bile-gut axis in the development and progression of intrahepatic cholangiocarcinoma. Bile-gut axis refers to the complex interaction between bile and gut microbiota, including bile salt metabolism, dynamic changes of microbiota, inflammatory response, and immune system regulation. This article elaborates on the potential mechanisms of bile-gut axis in intrahepatic cholangiocarcinoma, especially gut microbiota dysbiosis, abnormal bile salt metabolism, chronic inflammatory response, and immune system interaction, this article aims to provide new perspectives and possible therapeutic targets for future research and promote the early diagnosis and effective treatment of intrahepatic cholangiocarcinoma.
-
Key words:
- Cholangiocarcinoma /
- Bile-Gut Axis /
- Gastrointestinal Microbiome /
- Bile Salt Metabolism /
- Inflammation
-
[1] FERLAY J, COLOMBET M, SOERJOMATARAM I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer, 2019, 144( 8): 1941- 1953. DOI: 10.1002/ijc.31937. [2] AN L, ZHENG RS, ZHANG SW, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: Estimates based on data from 188 population-based cancer registries[J]. Hepatobiliary Surg Nutr, 2023, 12( 1): 45- 55. DOI: 10.21037/hbsn-21-75. [3] SIEGEL RL, MILLER KD, WAGLE NS, et al. Cancer statistics, 2023[J]. CA A Cancer J Clin, 2023, 73( 1): 17- 48. DOI: 10.3322/caac.21763. [4] WEI WQ, ZENG HM, ZHENG RS, et al. Cancer registration in China and its role in cancer prevention and control[J]. Lancet Oncol, 2020, 21( 7): e342- e349. DOI: 10.1016/S1470-2045(20)30073-5. [5] KHAN AS, DAGEFORDE LA. Cholangiocarcinoma[J]. Surg Clin North Am, 2019, 99( 2): 315- 335. DOI: 10.1016/j.suc.2018.12.004. [6] KNITTER S, RASCHZOK N, HILLEBRANDT KH, et al. Short-term postoperative outcomes of lymphadenectomy for cholangiocarcinoma, hepatocellular carcinoma and colorectal liver metastases in the modern era of liver surgery: Insights from the StuDoQ|Liver registry[J]. Eur J Surg Oncol, 2024, 50( 4): 108010. DOI: 10.1016/j.ejso.2024.108010. [7] LI ML, LIU FT, ZHANG F, et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: A whole-exome sequencing analysis[J]. Gut, 2019, 68( 6): 1024- 1033. DOI: 10.1136/gutjnl-2018-316039. [8] SAHU S, SUN WJ. Targeted therapy in biliary tract cancers-current limitations and potentials in the future[J]. J Gastrointest Oncol, 2017, 8( 2): 324- 336. DOI: 10.21037/jgo.2016.09.16. [9] TOLEDO B, DEIANA C, SCIANÒ F, et al. Treatment resistance in pancreatic and biliary tract cancer: Molecular and clinical pharmacology perspectives[J]. Expert Rev Clin Pharmacol, 2024, 17( 4): 323- 347. DOI: 10.1080/17512433.2024.2319340. [10] COSGROVE DP, REESE ES, FULCHER NM, et al. Real-world outcomes among patients with advanced or metastatic biliary tract cancers initiating second-line treatment[J]. Cancer Med, 2023, 12( 4): 4195- 4205. DOI: 10.1002/cam4.5282. [11] SATO K, HAYASHI M, ABE K, et al. Pembrolizumab-induced sclerosing cholangitis in a lung adenocarcinoma patient with a remarkable response to chemotherapy: A case report[J]. Clin J Gastroenterol, 2020, 13( 6): 1310- 1314. DOI: 10.1007/s12328-020-01178-5. [12] MAWSON AR, CROFT AM, GONZALEZ-FERNANDEZ F. Liver damage and exposure to toxic concentrations of endogenous retinoids in the pathogenesis of COVID-19 disease: Hypothesis[J]. Viral Immunol, 2021, 34( 6): 376- 379. DOI: 10.1089/vim.2020.0330. [13] HU YF, LI SX, LIU HL, et al. Precirrhotic primary biliary cholangitis with portal hypertension: Bile duct injury correlate[J]. Gut Liver, 2024, 18( 5): 867- 876. DOI: 10.5009/gnl230468. [14] XIE LJ, RUAN DD, ZHANG JH, et al. Mutational analysis of a familial adenomatous polyposis pedigree with bile duct polyp phenotype[J]. Can J Gastroenterol Hepatol, 2021, 2021: 6610434. DOI: 10.1155/2021/6610434. [15] OVERI D, CARPINO G, CRISTOFERI L, et al. Role of ductular reaction and ductular-canalicular junctions in identifying severe primary biliary cholangitis[J]. JHEP Rep, 2022, 4( 11): 100556. DOI: 10.1016/j.jhepr.2022.100556. [16] LIN CA, WANG YW, LIU CY, et al. Regulatory T cells in inflamed liver are dysfunctional in murine primary biliary cholangitis[J]. Clin Exp Immunol, 2024, 215( 3): 225- 239. DOI: 10.1093/cei/uxad117. [17] ARREAZA-GIL V, ESCOBAR-MARTÍNEZ I, MUGUERZA B, et al. The effects of grape seed proanthocyanidins in Cafeteria diet-induced obese Fischer 344 rats are influenced by faecal microbiota in a photoperiod dependent manner[J]. Food Funct, 2022, 13( 16): 8363- 8374. DOI: 10.1039/d2fo01206e. [18] FARSHBAFNADI M, AGAH E, REZAEI N. The second brain: The connection between gut microbiota composition and multiple sclerosis[J]. J Neuroimmunol, 2021, 360: 577700. DOI: 10.1016/j.jneuroim.2021.577700. [19] SOTTAS C, SCHMIEDOVÁ L, KREISINGER J, et al. Gut microbiota in two recently diverged passerine species: Evaluating the effects of species identity, habitat use and geographic distance[J]. BMC Ecol Evol, 2021, 21( 1): 41. DOI: 10.1186/s12862-021-01773-1. [20] GU SM, XIE Q, CHEN C, et al. Gut microbial signatures associated with peanut allergy in a BALB/c mouse model[J]. Foods, 2022, 11( 10): 1395. DOI: 10.3390/foods11101395. [21] KIM JK, CHOI MS, KIM JY, et al. Ginkgo biloba leaf extract suppresses intestinal human breast cancer resistance protein expression in mice: Correlation with gut microbiota[J]. Biomed Pharmacother, 2021, 140: 111712. DOI: 10.1016/j.biopha.2021.111712. [22] VERHAAR BJH, MOSTERD CM, COLLARD D, et al. Sex differences in associations of plasma metabolites with blood pressure and heart rate variability: The HELIUS study[J]. Atherosclerosis, 2023, 384: 117147. DOI: 10.1016/j.atherosclerosis.2023.05.016. [23] MIHAJLOVIC J, LEUTNER M, HAUSMANN B, et al. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women[J]. Environ Microbiol, 2021, 23( 6): 3037- 3047. DOI: 10.1111/1462-2920.15517. [24] SAAB M, MESTIVIER D, SOHRABI M, et al. Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma[J]. PLoS One, 2021, 16( 3): e0247798. DOI: 10.1371/journal.pone.0247798. [25] LIU XJ, CHENG Y, ZANG D, et al. The role of gut microbiota in lung cancer: From carcinogenesis to immunotherapy[J]. Front Oncol, 2021, 11: 720842. DOI: 10.3389/fonc.2021.720842. [26] LI T, WANG PL, YUAN ZB, et al. Changes in intestinal flora in patients with extrahepatic cholangiocarcinoma[J]. J Clin Hepatol, 2021, 37( 12): 2883- 2889. DOI: 10.3969/j.issn.1001-5256.2021.12.029.李涛, 王盼梁, 袁梓博, 等. 肝外肝内胆管癌患者肠道菌群变化分析[J]. 临床肝胆病杂志, 2021, 37( 12): 2883- 2889. DOI: 10.3969/j.issn.1001-5256.2021.12.029. [27] HUANG H, ZHONG W, WANG XJ, et al. The role of gastric microecological dysbiosis in gastric carcinogenesis[J]. Front Microbiol, 2023, 14: 1218395. DOI: 10.3389/fmicb.2023.1218395. [28] MADSEN C. The role of oral health in gastrointestinal malignancies[J]. J Gastrointest Oncol, 2021, 12( Suppl 2): S311- S315. DOI: 10.21037/jgo.2020.02.03. [29] LIU TY, GUO ZX, SONG XL, et al. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence[J]. J Cell Mol Med, 2020, 24( 4): 2648- 2662. DOI: 10.1111/jcmm.14984. [30] FAN XY, JIN YL, CHEN G, et al. Gut microbiota dysbiosis drives the development of colorectal cancer[J]. Digestion, 2021, 102( 4): 508- 515. DOI: 10.1159/000508328. [31] CUI H, LIAN J, XU BG, et al. Identification of a bile acid and bile salt metabolism-related lncRNA signature for predicting prognosis and treatment response in hepatocellular carcinoma[J]. Sci Rep, 2023, 13( 1): 19512. DOI: 10.1038/s41598-023-46805-6. [32] CAMILLERI M. Bile acid detergency: Permeability, inflammation, and effects of sulfation[J]. Am J Physiol Gastrointest Liver Physiol, 2022, 322( 5): G480- G488. DOI: 10.1152/ajpgi.00011.2022. [33] WANG N, YU XH, XU L. Intestinal flora and cholangiocarcinoma: Research progress[J]. Chin J Microecol, 2018, 30( 11): 1339- 1342. DOI: 10.13381/j.cnki.cjm.201811024.王宁, 于兴华, 许琳. 肠道菌群的研究进展及与肝内胆管癌的关系分析[J]. 中国微生态学杂志, 2018, 30( 11): 1339- 1342. DOI: 10.13381/j.cnki.cjm.201811024. [34] BRUNEAU A, HUNDERTMARK J, GUILLOT A, et al. Molecular and cellular mediators of the gut-liver axis in the progression of liver diseases[J]. Front Med(Lausanne), 2021, 8: 725390. DOI: 10.3389/fmed.2021.725390. [35] WANG DH, ZHANG XS, DU HW. Inflammatory bowel disease: A potential pathogenic factor of Alzheimer’s disease[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2022, 119: 110610. DOI: 10.1016/j.pnpbp.2022.110610. [36] ZOU ZJ, LIN HF, LI MS, et al. Tumor-associated macrophage polarization in the inflammatory tumor microenvironment[J]. Front Oncol, 2023, 13: 1103149. DOI: 10.3389/fonc.2023.1103149. [37] LI LH, YU R, CAI TG, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment[J]. Int Immunopharmacol, 2020, 88: 106939. DOI: 10.1016/j.intimp.2020.106939. [38] SAHOO DK, BORCHERDING DC, CHANDRA L, et al. Differential transcriptomic profiles following stimulation with lipopolysaccharide in intestinal organoids from dogs with inflammatory bowel disease and intestinal mast cell tumor[J]. Cancers(Basel), 2022, 14( 14): 3525. DOI: 10.3390/cancers14143525. [39] VIGNJEVIĆ PETRINOVIĆ S, MILOŠEVIĆ MS, MARKOVIĆ D, et al. Interplay between stress and cancer-a focus on inflammation[J]. Front Physiol, 2023, 14: 1119095. DOI: 10.3389/fphys.2023.1119095. [40] AL-RAJABI R, SUN WJ. Immunotherapy in cholangiocarcinoma[J]. Curr Opin Gastroenterol, 2021, 37( 2): 105- 111. DOI: 10.1097/MOG.0000000000000715. [41] WITSCHEN PM, CHAFFEE TS, BRADY NJ, et al. Tumor cell associated hyaluronan-CD44 signaling promotes pro-tumor inflammation in breast cancer[J]. Cancers(Basel), 2020, 12( 5): 1325. DOI: 10.3390/cancers12051325. [42] TAN ZF, XUE HB, SUN YL, et al. The role of tumor inflammatory microenvironment in lung cancer[J]. Front Pharmacol, 2021, 12: 688625. DOI: 10.3389/fphar.2021.688625. [43] LIU GL, DOU J, MENG HJ, et al. Value of immune-inflammatory factors in predicting intrahepatic cholangiocarcinoma[J]. J Clin Hepatol, 2023, 39( 9): 2231- 2236. DOI: 10.3969/j.issn.1001-5256.2023.09.030.刘桂玲, 窦杰, 孟慧娟, 等. 免疫炎症因子在肝内肝内胆管癌中的预测价值[J]. 临床肝胆病杂志, 2023, 39( 9): 2231- 2236. DOI: 10.3969/j.issn.1001-5256.2023.09.030. [44] SCIMECA M, ROVELLA V, PALUMBO V, et al. Programmed cell death pathways in cholangiocarcinoma: Opportunities for targeted therapy[J]. Cancers(Basel), 2023, 15( 14): 3638. DOI: 10.3390/cancers15143638. [45] CHEN S, WANG J. Advances in tumor microenvironment and immunotherapy of Cholangiocarcinoma[J]. J Clin Hepatol, 2022, 38( 10): 2428- 2432. DOI: 10.3969/j.issn.1001-5256.2022.10.044.陈顺, 王俊. 肝内胆管癌肿瘤微环境与免疫治疗[J]. 临床肝胆病杂志, 2022, 38( 10): 2428- 2432. DOI: 10.3969/j.issn.1001-5256.2022.10.044. [46] XIA T, LI KY, NIU N, et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses[J]. J Hematol Oncol, 2022, 15( 1): 37. DOI: 10.1186/s13045-022-01253-z. [47] ALVISI G, TERMANINI A, SOLDANI C, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target[J]. J Hepatol, 2022, 77( 5): 1359- 1372. DOI: 10.1016/j.jhep.2022.05.043. [48] ZHANG ZJ, HUANG YP, LIU ZT, et al. Identification of immune related gene signature for predicting prognosis of cholangiocarcinoma patients[J]. Front Immunol, 2023, 14: 1028404. DOI: 10.3389/fimmu.2023.1028404. [49] ZHOU GY, LIESHOUT R, van TIENDEREN GS, et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells[J]. Br J Cancer, 2022, 127( 4): 649- 660. DOI: 10.1038/s41416-022-01839-x. [50] GRETEN TF, SCHWABE R, BARDEESY N, et al. Immunology and immunotherapy of cholangiocarcinoma[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 6): 349- 365. DOI: 10.1038/s41575-022-00741-4. [51] CAZZETTA V, FRANZESE S, CARENZA C, et al. Natural killer-dendritic cell interactions in liver cancer: Implications for immunotherapy[J]. Cancers(Basel), 2021, 13( 9): 2184. DOI: 10.3390/cancers13092184. [52] CHEN ZM, CHEN JH. Advances in precision diagnosis and treatment of cholangiocarcinoma[J]. Chin J Clin Pharmacol Ther, 2025, 30( 2): 159- 170. DOI: 10.12092/j.issn.1009-2501.2025.02.002.陈祯美, 陈进宏. 肝内胆管癌精准诊疗进展及前沿[J]. 中国临床药理学与治疗学, 2025, 30( 2): 159- 170. DOI: 10.12092/j.issn.1009-2501.2025.02.002. [53] YU XZ, ZHU LL, WANG T, et al. Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies[J]. Front Immunol, 2023, 14: 1037945. DOI: 10.3389/fimmu.2023.1037945. [54] CHIANG NJ, HOU YC, TAN KT, et al. The immune microenvironment features and response to immunotherapy in EBV-associated lymphoepithelioma-like cholangiocarcinoma[J]. Hepatol Int, 2022, 16( 5): 1137- 1149. DOI: 10.1007/s12072-022-10346-3. [55] KIDA A, MIZUKOSHI E, KITAHARA M, et al. Effects of adoptive T-cell immunotherapy on immune cell profiles and prognosis of patients with unresectable or recurrent cholangiocarcinoma[J]. Int J Cancer, 2024, 154( 4): 738- 747. DOI: 10.1002/ijc.34716. -



 PDF下载 ( 1044 KB)
PDF下载 ( 1044 KB)

 下载:
下载:


