| [1] |
SCHNABL B. PPAR agonists in primary biliary cholangitis[J]. N Engl J Med, 2024, 390( 9): 855- 858. DOI: 10.1056/nejme2313802. |
| [2] |
Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management of primary biliary cholangitis(2021)[J]. J Clin Hepatol, 2022, 38( 1): 35- 41.
中华医学会肝病学分会. 原发性胆汁性胆管炎的诊断和治疗指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 35- 41.
|
| [3] |
HARMS MH, van BUUREN HR, CORPECHOT C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis[J]. J Hepatol, 2019, 71( 2): 357- 365. DOI: 10.1016/j.jhep.2019.04.001. |
| [4] |
European Association for the Study of the Liver. EASL clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis[J]. J Hepatol, 2017, 67( 1): 145- 172. DOI: 10.1016/j.jhep.2017.03.022. |
| [5] |
YOU H, MA X, EFE C, et al. APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis[J]. Hepatol Int, 2022, 16( 1): 1- 23. DOI: 10.1007/s12072-021-10276-6. |
| [6] |
ZANDANELL S, STRASSER M, FELDMAN A, et al. Similar clinical outcome of AMA immunoblot-M2-negative compared to immunoblot-positive subjects over six years of follow-up[J]. Postgrad Med, 2021, 133( 3): 291- 298. DOI: 10.1080/00325481.2021.1885945. |
| [7] |
HALDAR D, JANMOHAMED A, PLANT T, et al. Antibodies to gp210 and understanding risk in patients with primary biliary cholangitis[J]. Liver Int, 2021, 41( 3): 535- 544. DOI: 10.1111/liv.14688. |
| [8] |
REIG A, NORMAN GL, GARCIA M, et al. Novel anti-hexokinase 1 antibodies are associated with poor prognosis in patients with primary biliary cholangitis[J]. Am J Gastroenterol, 2020, 115( 10): 1634- 1641. DOI: 10.14309/ajg.0000000000000690. |
| [9] |
NAKAMURA M, KONDO H, MORI T, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis[J]. Hepatology, 2007, 45( 1): 118- 127. DOI: 10.1002/hep.21472. |
| [10] |
LAMMERS WJ, HIRSCHFIELD GM, CORPECHOT C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy[J]. Gastroenterology, 2015, 149( 7): 1804- 1812. DOI: 10.1053/j.gastro.2015.07.061. |
| [11] |
YANG F, YANG Y, WANG Q, et al. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: The additional effect of anti-gp210[J]. Aliment Pharmacol Ther, 2017, 45( 5): 733- 743. DOI: 10.1111/apt.13927. |
| [12] |
CHANG YH, WANG L, YUAN Z, et al. Application and evaluation of prognostic value of continuous scoring systems in different stages of primary biliary cholangitis patients in China[J]. Int J Dig Dis, 2019, 39( 2): 102- 110. DOI: 10.3969/j.issn.1673-534X.2019.02.008. |
| [13] |
LAMMERS WJ, van BUUREN HR, HIRSCHFIELD GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study[J]. Gastroenterology, 2014, 147( 6): 1338- 1349. DOI: 10.1053/j.gastro.2014.08.029. |
| [14] |
HIRSCHFIELD GM, BEUERS U, KUPCINSKAS L, et al. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA[J]. J Hepatol, 2021, 74( 2): 321- 329. DOI: 10.1016/j.jhep.2020.09.011. |
| [15] |
NEVENS F, ANDREONE P, MAZZELLA G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis[J]. N Engl J Med, 2016, 375( 7): 631- 643. DOI: 10.1056/NEJMoa1509840. |
| [16] |
|
| [17] |
HOURI I, HIRSCHFIELD GM. Primary biliary cholangitis: Pathophysiology[J]. Clin Liver Dis, 2024, 28( 1): 79- 92. DOI: 10.1016/j.cld.2023.06.006. |
| [18] |
TANAKA A. Current understanding of primary biliary cholangitis[J]. Clin Mol Hepatol, 2021, 27( 1): 1- 21. DOI: 10.3350/cmh.2020.0028. |
| [19] |
SAKUGAWA H, NAKASONE H, NAKAYOSHI T, et al. Epidemiology of primary biliary cirrhosis among women with elevated gamma-glutamyl transpeptidase levels in Okinawa, Japan[J]. Hepatol Res, 2003, 26( 4): 330- 336. DOI: 10.1016/s1386-6346(03)00167-0. |
| [20] |
LIU ZC, WANG ZL, ZHENG JR, et al. Prevalence of primary biliary cholangitis in the Chinese general population and its influencing factors: A systematic review[J]. J Clin Hepatol, 2023, 39( 2): 325- 332. DOI: 10.3969/j.issn.1001-5256.2023.02.011. |
| [21] |
HUANG CY, LIU YM, LIU H, et al. Study of clinical characteristics in patients with gp210 antibody-positive primary biliary cholangitis[J]. Chin J Hepatol, 2022, 30( 4): 419- 425. DOI: 10.3760/cma.j.cn501113-20210501-00216. |
| [22] |
WANG J, XIA J, YAN XM, et al. Plateletcrit as a potential index for predicting liver fibrosis in chronic hepatitis B[J]. J Viral Hepat, 2020, 27( 6): 602- 609. DOI: 10.1111/jvh.13264. |
| [23] |
LIU YW, HAN K, LIU C, et al. Clinical characteristics and prognosis of concomitant primary biliary cholangitis and autoimmune diseases: A retrospective study[J]. Can J Gastroenterol Hepatol, 2021, 2021: 5557814. DOI: 10.1155/2021/5557814. |
| [24] |
HU SL, ZHAO FR, HU Q, et al. Meta-analysis assessment of GP210 and SP100 for the diagnosis of primary biliary cirrhosis[J]. PLoS One, 2014, 9( 7): e101916. DOI: 10.1371/journal.pone.0101916. |
| [25] |
LINDOR KD, BOWLUS CL, BOYER J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases[J]. Hepatology, 2019, 69( 1): 394- 419. DOI: 10.1002/hep.30145. |
| [26] |
MYTILINAIOU MG, MEYER W, SCHEPER T, et al. Diagnostic and clinical utility of antibodies against the nuclear body promyelocytic leukaemia and Sp100 antigens in patients with primary biliary cirrhosis[J]. Clin Chim Acta, 2012, 413( 15-16): 1211- 1216. DOI: 10.1016/j.cca.2012.03.020. |
| [27] |
NAKAMURA M, TAKII Y, ITO M, et al. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis[J]. J Autoimmun, 2006, 26( 2): 138- 145. DOI: 10.1016/j.jaut.2005.10.007. |



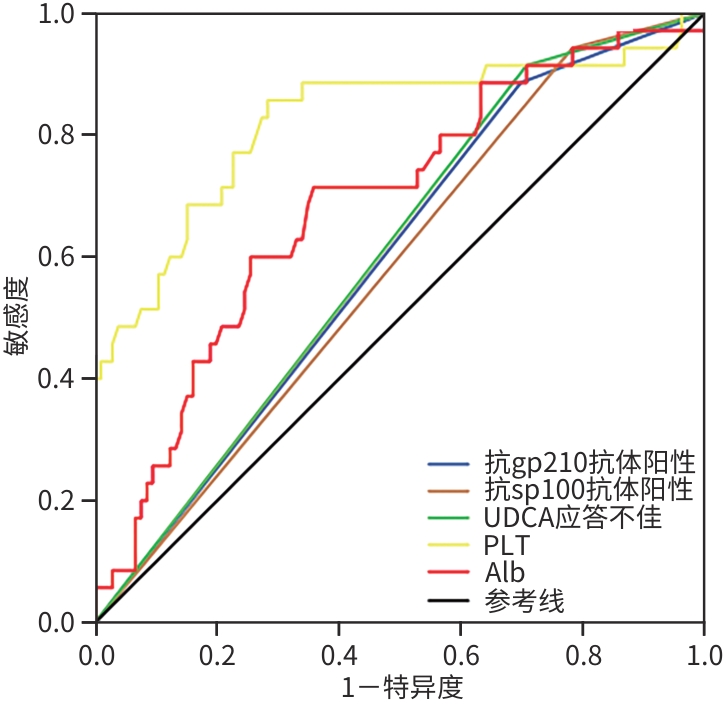




 DownLoad:
DownLoad: