| [1] |
ZHAO J, LIU L, CAO YY, et al. MAFLD as part of systemic metabolic dysregulation[J]. Hepatol Int, 2024, 18( Suppl 2): 834- 847. DOI: 10.1007/s12072-024-10660-y. |
| [2] |
LOU TW, YANG RX, FAN JG. The global burden of fatty liver disease: The major impact of China[J]. Hepatobiliary Surg Nutr, 2024, 13( 1): 119- 123. DOI: 10.21037/hbsn-23-556. |
| [3] |
YOUNOSSI ZM, KALLIGEROS M, HENRY L. Epidemiology of metabolic dysfunction-associated steatotic liver disease[J]. Clin Mol Hepatol, 2025, 31( Suppl): S32- S50. DOI: 10.3350/cmh.2024.0431. |
| [4] |
NAM D, CHAPIRO J, PARADIS V, et al. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction[J]. JHEP Rep, 2022, 4( 4): 100443. DOI: 10.1016/j.jhepr.2022.100443. |
| [5] |
CADDEO A, ROMEO S. Precision medicine and nucleotide-based therapeutics to treat steatotic liver disease[J]. Clin Mol Hepatol, 2025, 31( Suppl): S76- S93. DOI: 10.3350/cmh.2024.0438. |
| [6] |
RATZIU V, FRIEDMAN SL. Why do so many nonalcoholic steatohepatitis trials fail?[J]. Gastroenterology, 2023, 165( 1): 5- 10. DOI: 10.1053/j.gastro.2020.05.046. |
| [7] |
ABDELHAMEED F, KITE C, LAGOJDA L, et al. Non-invasive scores and serum biomarkers for fatty liver in the era of metabolic dysfunction-associated steatotic liver disease(MASLD): A comprehensive review from NAFLD to MAFLD and MASLD[J]. Curr Obes Rep, 2024, 13( 3): 510- 531. DOI: 10.1007/s13679-024-00574-z. |
| [8] |
MIAO L, TARGHER G, BYRNE CD, et al. Current status and future trends of the global burden of MASLD[J]. Trends Endocrinol Metab, 2024, 35( 8): 697- 707. DOI: 10.1016/j.tem.2024.02.007. |
| [9] |
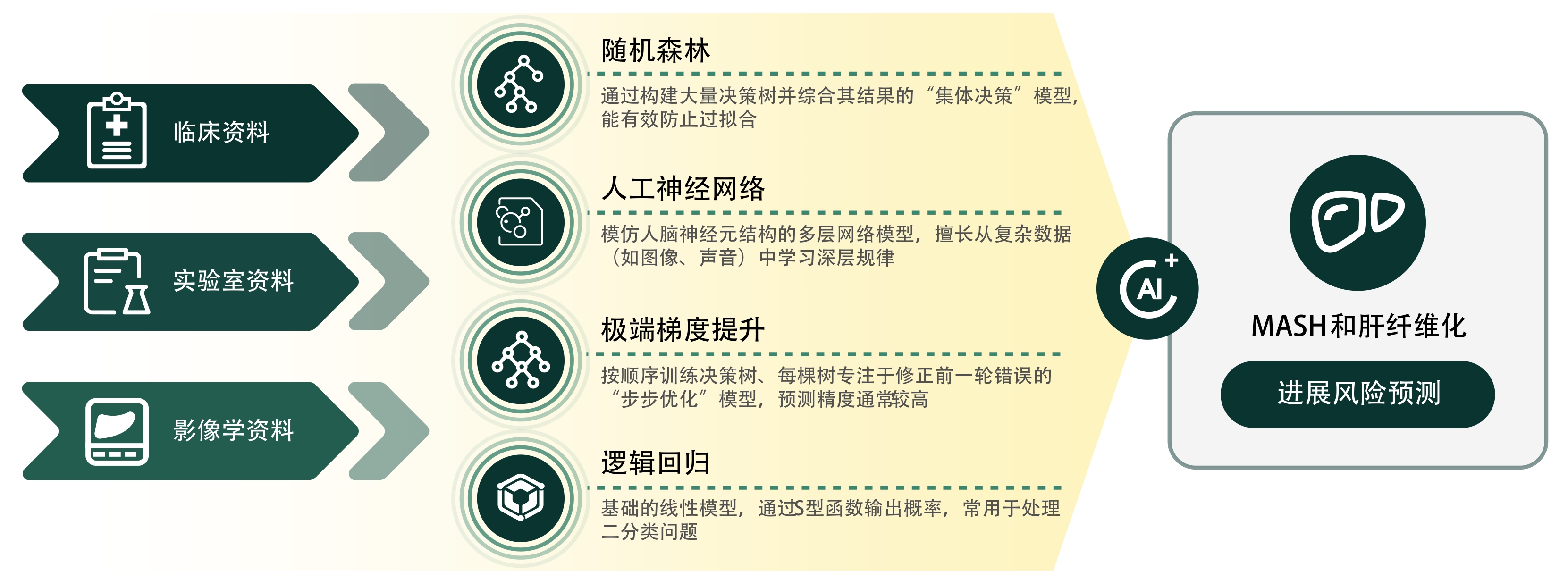
DENG JL, JI WD, LIU HZ, et al. Development and validation of a machine learning-based framework for assessing metabolic-associated fatty liver disease risk[J]. BMC Public Health, 2024, 24( 1): 2545. DOI: 10.1186/s12889-024-19882-z. |
| [10] |
HUANG GQ, JIN QK, MAO YS. Predicting the 5-year risk of nonalcoholic fatty liver disease using machine learning models: Prospective cohort study[J]. J Med Internet Res, 2023, 25: e46891. DOI: 10.2196/46891. |
| [11] |
DESTREMPES F, GESNIK M, CHAYER B, et al. Quantitative ultrasound, elastography, and machine learning for assessment of steatosis, inflammation, and fibrosis in chronic liver disease[J]. PLoS One, 2022, 17( 1): e0262291. DOI: 10.1371/journal.pone.0262291. |
| [12] |
ALSHAGATHRH FM, HOUSEH MS. Artificial intelligence for detecting and quantifying fatty liver in ultrasound images: A systematic review[J]. Bioengineering, 2022, 9( 12): 748. DOI: 10.3390/bioengineering9120748. |
| [13] |
PICKHARDT PJ, BLAKE GM, GRAFFY PM, et al. Liver steatosis categorization on contrast-enhanced CT using a fully automated deep learning volumetric segmentation tool: Evaluation in 1204 healthy adults using unenhanced CT as a reference standard[J]. AJR Am J Roentgenol, 2021, 217( 2): 359- 367. DOI: 10.2214/AJR.20.24415. |
| [14] |
CHOI KJ, JANG JK, LEE SS, et al. Development and validation of a deep learning system for staging liver fibrosis by using contrast agent-enhanced CT images in the liver[J]. Radiology, 2018, 289( 3): 688- 697. DOI: 10.1148/radiol.2018180763. |
| [15] |
YASAKA K, AKAI H, KUNIMATSU A, et al. Liver fibrosis: Deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images[J]. Radiology, 2018, 287( 1): 146- 155. DOI: 10.1148/radiol.2017171928. |
| [16] |
ZARELLA MD, BOWMAN D, AEFFNER F, et al. A practical guide to whole slide imaging: A white paper from the digital pathology association[J]. Arch Pathol Lab Med, 2019, 143( 2): 222- 234. DOI: 10.5858/arpa.2018-0343-RA. |
| [17] |
TAYLOR-WEINER A, POKKALLA H, HAN L, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH[J]. Hepatology, 2021, 74( 1): 133- 147. DOI: 10.1002/hep.31750. |
| [18] |
ASTBURY S, GROVE JI, DORWARD DA, et al. Reliable computational quantification of liver fibrosis is compromised by inherent staining variation[J]. J Pathol Clin Res, 2021, 7( 5): 471- 481. DOI: 10.1002/cjp2.227. |
| [19] |
LEOW WQ, BEDOSSA P, LIU F, et al. An improved qFibrosis algorithm for precise screening and enrollment into non-alcoholic steatohepatitis(NASH) clinical trials[J]. Diagnostics, 2020, 10( 9): 643. DOI: 10.3390/diagnostics10090643. |
| [20] |
BOSCH J, CHUNG C, CARRASCO-ZEVALLOS OM, et al. A machine learning approach to liver histological evaluation predicts clinically significant portal hypertension in NASH cirrhosis[J]. Hepatology, 2021, 74( 6): 3146- 3160. DOI: 10.1002/hep.32087. |
| [21] |
RATZIU V, HOMPESCH M, PETITJEAN M, et al. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: Current status and future directions[J]. J Hepatol, 2024, 80( 2): 335- 351. DOI: 10.1016/j.jhep.2023.10.015. |
| [22] |
NJEI B, OSTA E, NJEI N, et al. An explainable machine learning model for prediction of high-risk nonalcoholic steatohepatitis[J]. Sci Rep, 2024, 14( 1): 8589. DOI: 10.1038/s41598-024-59183-4. |
| [23] |
MUNK LAURIDSEN M, RAVNSKJAER K, GLUUD LL, et al. Disease classification, diagnostic challenges, and evolving clinical trial design in MASLD[J]. J Clin Invest, 2025, 135( 10): e189953. DOI: 10.1172/JCI189953. |
| [24] |
CHANG D, TRUONG E, MENA EA, et al. Machine learning models are superior to noninvasive tests in identifying clinically significant stages of NAFLD and NAFLD-related cirrhosis[J]. Hepatology, 2023, 77( 2): 546- 557. DOI: 10.1002/hep.32655. |
| [25] |
KWON OY, CHOI JY, JANG Y. The effectiveness of eHealth interventions on lifestyle modification in patients with nonalcoholic fatty liver disease: Systematic review and meta-analysis[J]. J Med Internet Res, 2023, 25: e37487. DOI: 10.2196/37487. |
| [26] |
TINCOPA MA, PATEL N, SHAHAB A, et al. Implementation of a randomized mobile-technology lifestyle program in individuals with nonalcoholic fatty liver disease[J]. Sci Rep, 2024, 14( 1): 7452. DOI: 10.1038/s41598-024-57722-7. |
| [27] |
TINCOPA MA, LYDEN A, WONG J, et al. Impact of a pilot structured mobile technology based lifestyle intervention for patients with nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2022, 67( 2): 481- 491. DOI: 10.1007/s10620-021-06922-6. |
| [28] |
SATO M, AKAMATSU M, SHIMA T, et al. Impact of a novel digital therapeutics system on nonalcoholic steatohepatitis: The NASH app clinical trial[J]. Am J Gastroenterol, 2023, 118( 8): 1365- 1372. DOI: 10.14309/ajg.0000000000002143. |
| [29] |
KWON OY, LEE MK, LEE HW, et al. Mobile app-based lifestyle coaching intervention for patients with nonalcoholic fatty liver disease: Randomized controlled trial[J]. J Med Internet Res, 2024, 26: e49839. DOI: 10.2196/49839. |







 DownLoad:
DownLoad: