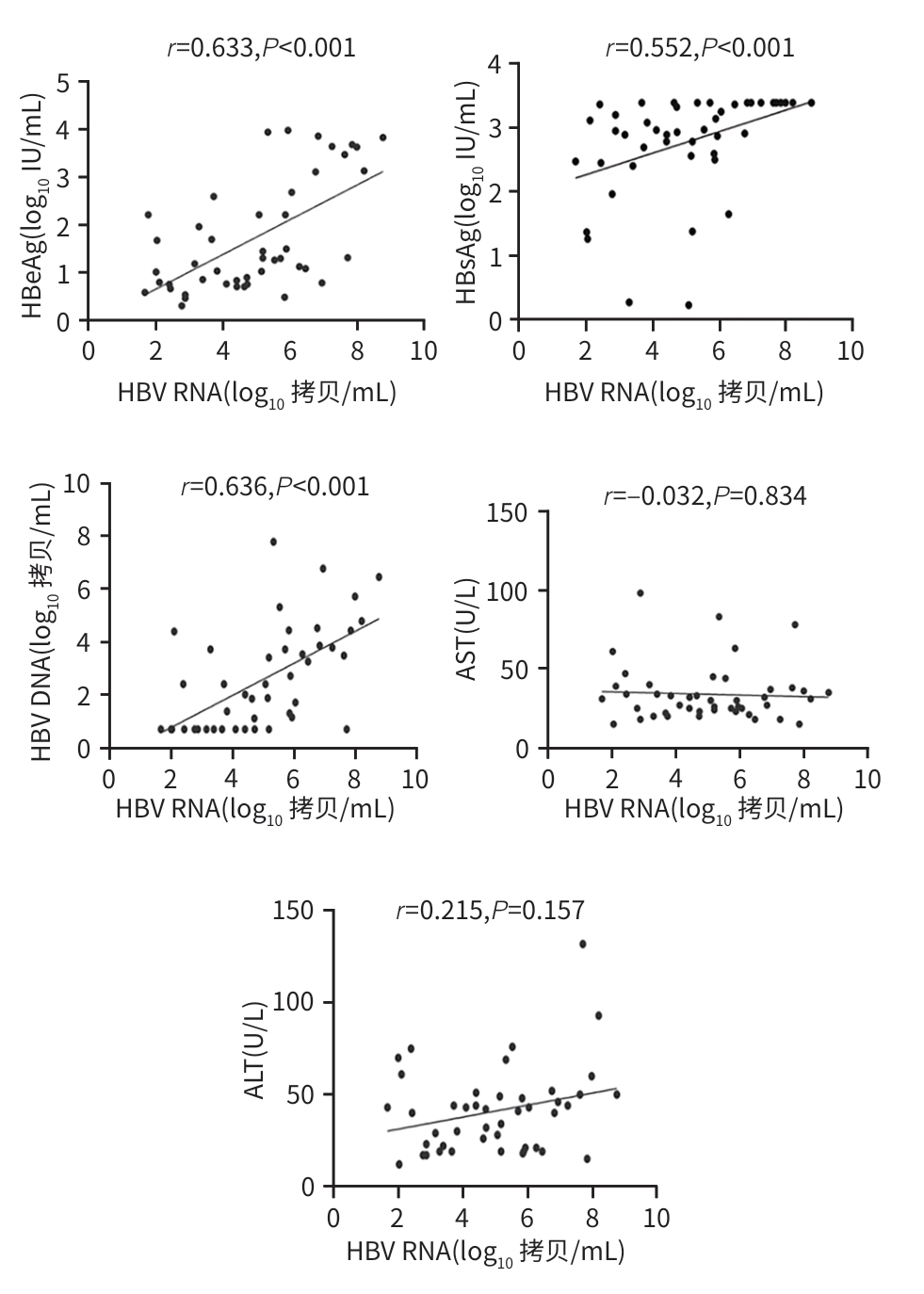
| [1] |
GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 796- 829. DOI: 10.1016/s2468-1253(22)00124-8. |
| [2] |
ZHANG SH, CUI FQ. Global progress, challenges and strategies in eliminating public threat of viral hepatitis[J]. Infect Dis Poverty, 2025, 14( 1): 9. DOI: 10.1186/s40249-025-01275-y. |
| [3] |
WANG J, ZHANG S, ZHU C, et al. Treatment coverage of the 2024 updated WHO guidelines for patients with chronic hepatitis B[J]. J Hepatol, 2025, 82( 6): e309- e310. DOI: 10.1016/j.jhep.2025.01.039. |
| [4] |
|
| [5] |
HAN GR, JIANG HX. New advances in antiviral therapy during pregnancy to block mother-to-child transmission of hepatitis B virus[J]. J Clin Hepatol, 2024, 40( 11): 2158- 2163. DOI: 10.12449/JCH241105. 韩国荣, 江红秀. 妊娠期抗病毒治疗阻断HBV母婴传播的新进展[J]. 临床肝胆病杂志, 2024, 40( 11): 2158- 2163. DOI: 10.12449/JCH241105. |
| [6] |
ZHUANG H. Progress towards elimination of hepatitis B[J]. J Clin Hepatol, 2024, 40( 5): 857- 860. DOI: 10.12449/JCH240501. |
| [7] |
Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. |
| [8] |
GIERSCH K, ALLWEISS L, VOLZ T, et al. Serum HBV pgRNA as a clinical marker for cccDNA activity[J]. J Hepatol, 2017, 66( 2): 460- 462. DOI: 10.1016/j.jhep.2016.09.028. |
| [9] |
TANG LSY, COVERT E, WILSON E, et al. Chronic hepatitis B infection: A review[J]. JAMA, 2018, 319( 17): 1802- 1813. DOI: 10.1001/jama.2018.3795. |
| [10] |
ZHANG SL, CAO MM, YANG F, et al. Analysis of the change trend of etiological burden of disease of liver cancer in the Chinese population from 1990 to 2019[J]. Chin J Dig Surg, 2023, 22( 1): 122- 130. DOI: 10.3760/cma.j.cn115610-20221112-00687. |
| [11] |
BOONSTRA A, SARI G. HBV cccDNA: The molecular reservoir of hepatitis B persistence and challenges to achieve viral eradication[J]. Biomolecules, 2025, 15( 1): 62. DOI: 10.3390/biom15010062. |
| [12] |
YEH SH, LI CL, LIN YY, et al. Hepatitis B virus DNA integration drives carcinogenesis and provides a new biomarker for HBV-related HCC[J]. Cell Mol Gastroenterol Hepatol, 2023, 15( 4): 921- 929. DOI: 10.1016/j.jcmgh.2023.01.001. |
| [13] |
DENG R, LIU S, SHEN S, et al. Circulating HBV RNA: From biology to clinical applications[J]. Hepatology, 2022, 76( 5): 1520- 1530. DOI: 10.1002/hep.32479. |
| [14] |
HE XJ, LONG YZ, ZHOU J, et al. Serum hepatitis B virus RNA monitoring pegylated interferon therapy nucleos(t)ide analogues in the treatment of low viral load in patients with chronic hepatitis B curative effect[J]. Clin J Med Offic, 2023, 51( 10): 1091- 1095. DOI: 10.16680/j.16713-826.2023.10.27.
|
| [15] |
FARAG MS, van CAMPENHOUT MJH, PFEFFERKORN M, et al. Hepatitis B virus RNA as early predictor for response to pegylated interferon alpha in HBeAg-negative chronic hepatitis B[J]. Clin Infect Dis, 2021, 72( 2): 202- 211. DOI: 10.1093/cid/ciaa013. |
| [16] |
MAHAJAN A, KHARAWALA S, DESAI S, et al. Association of hepatitis B surface antigen levels with long-term complications in chronic hepatitis B virus infection: A systematic literature review[J]. J Viral Hepat, 2024, 31( 11): 746- 759. DOI: 10.1111/jvh.13988. |
| [17] |
ZHOU LL, DONG B, XIN JJ, et al. Liver histopathological features of HBeAg-negative patients in the indeterminate phase of low-viral-load chronic hepatitis B virus infection[J]. J Clin Hepatol, 2025, 41( 1): 52- 56. DOI: 10.12449/JCH250108. 周路路, 东冰, 辛杰晶, 等. 低病毒载量HBeAg阴性不确定期慢性HBV感染者肝组织病理分析[J]. 临床肝胆病杂志, 2025, 41( 1): 52- 56. DOI: 10.12449/JCH250108. |
| [18] |
CAI G, GAO QE. Relationships between changes in serum HBV RNA levels and HBeAg positivity and cirrhosis in patients with CHB during antiviral therapy[J]. Shandong Med J, 2025, 65( 1): 104- 108. DOI: 10.3969/j.issn.1002-266X.2025.01.022. |
| [19] |
JIN MH, JIANG SW, HU AR, et al. Research progress on differential improvement and mechanism of nucleoside analogues or nucleotide analogues in HBV-related hepatocellular carcinoma[J]. Chin J Clin Pharmacol Ther, 2025, 30( 6): 835- 848. DOI: 10.12092/j.issn.1009-2501.2025.06.014. |
| [20] |
LI FH, QU LH, LIU YH, et al. PegIFN alpha-2a reduces relapse in HBeAg-negative patients after nucleo(s)tide analogue cessation: A randomized-controlled trial[J]. J Hepatol, 2025, 82( 2): 211- 221. DOI: 10.1016/j.jhep.2024.07.019. |
| [21] |
JANSSEN HLA, SONNEVELD MJ. Combination therapy for chronic HBV infection[J]. N Engl J Med, 2024, 391( 22): 2163- 2168. DOI: 10.1056/nejme2410543. |
| [22] |
WANG XM, CHI XM, WU RH, et al. Serum HBV RNA correlated with intrahepatic cccDNA more strongly than other HBV markers during peg-interferon treatment[J]. Virol J, 2021, 18( 1): 4. DOI: 10.1186/s12985-020-01471-2. |
| [23] |
YE F, ZHAO WJ, YANG XL, et al. The decline of hbv RNA associated with HBeAg seroconversion and double-negative hbv DNA and RNA in chronic hepatitis b patients who received entecavir therapy: A 10-year retrospective cohort study[J]. Ann Transl Med, 2022, 10( 16): 897. DOI: 10.21037/atm-22-3265. |
| [24] |
SONG GJ, YANG RF, JIN Q, et al. HBV pregenome RNA as a predictor of spontanous HBeAg seroconversion in HBeAg-positive chronic hepatitis B patients[J]. BMC Gastroenterol, 2023, 23( 1): 381. DOI: 10.1186/s12876-023-03023-8. |
| [25] |
WOODDELL CI, YUEN MF, CHAN HL, et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg[J]. Sci Transl Med, 2017, 9( 409): eaan0241. DOI: 10.1126/scitranslmed.aan0241. |
| [26] |
MAK LY, CLOHERTY G, WONG DK, et al. HBV RNA profiles in patients with chronic hepatitis B under different disease phases and antiviral therapy[J]. Hepatology, 2021, 73( 6): 2167- 2179. DOI: 10.1002/hep.31616. |
| [27] |
WANG X, TANG XQ, HAN N, et al. Research progress of biomarkers of hepatitis B virus and clinical significance[J]. J Biomed Eng, 2023, 40( 6): 1242- 1248. DOI: 10.7507/1001-5515.202309041. |



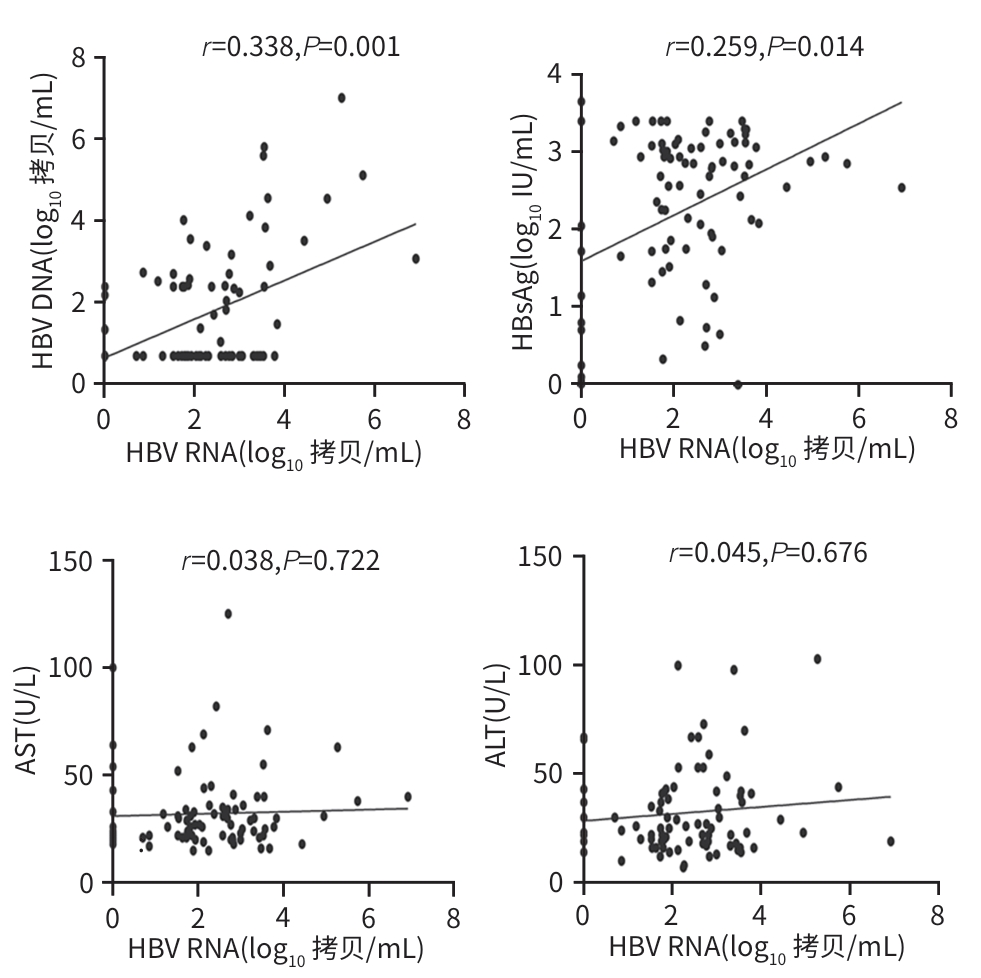




 DownLoad:
DownLoad: