| [1] |
STEFAN N, SCHICK F, BIRKENFELD AL, et al. The role of hepatokines in NAFLD[J]. Cell Metab, 2023, 35( 2): 236- 252. DOI: 10.1016/j.cmet.2023.01.006. |
| [2] |
MONSERRAT-MESQUIDA M, QUETGLAS-LLABRÉS M, BOUZAS C, et al. A greater improvement of intrahepatic fat contents after 6 months of lifestyle intervention is related to a better oxidative stress and inflammatory status in non-alcoholic fatty liver disease[J]. Antioxidants(Basel), 2022, 11( 7): 1266. DOI: 10.3390/antiox11071266. |
| [3] |
COTTER TG, RINELLA M. Nonalcoholic fatty liver disease 2020: The state of the disease[J]. Gastroenterology, 2020, 158( 7): 1851- 1864. DOI: 10.1053/j.gastro.2020.01.052. |
| [4] |
WU YK, ZHENG Q, ZOU BY, et al. The epidemiology of NAFLD in the Chinese mainland with analysis by adjusted gross regional domestic product: A meta-analysis[J]. Hepatol Int, 2020, 14( 2): 259- 269. DOI: 10.1007/s12072-020-10023-3. |
| [5] |
HUANG YZ, CHEN H, CHEN JL, et al. Yellow tea polysaccharides protect against non-alcoholic fatty liver disease via regulation of gut microbiota and bile acid metabolism in mice[J]. Phytomedicine, 2024, 133: 155919. DOI: 10.1016/j.phymed.2024.155919. |
| [6] |
JIANG HT, ZHU H, HUO GM, et al. Oudemansiella raphanipies polysaccharides improve lipid metabolism disorders in murine high-fat diet-induced non-alcoholic fatty liver disease[J]. Nutrients, 2022, 14( 19): 4092. DOI: 10.3390/nu14194092. |
| [7] |
LAN N, LU Y, ZHANG YG, et al. FTO-A common genetic basis for obesity and cancer[J]. Front Genet, 2020, 11: 559138. DOI: 10.3389/fgene.2020.559138. |
| [8] |
TAN J, WANG YF, DAI ZH, et al. Roles of RNA m6A modification in nonalcoholic fatty liver disease[J]. Hepatol Commun, 2023, 7( 2): e0046. DOI: 10.1097/HC9.0000000000000046. |
| [9] |
WEI XH, ZHANG JL, TANG M, et al. Fat mass and obesity-associated protein promotes liver steatosis by targeting PPARα[J]. Lipids Health Dis, 2022, 21: 29. DOI: 10.1186/s12944-022-01640-y. |
| [10] |
GAN XJ, DAI ZH, GE CM, et al. FTO promotes liver inflammation by suppressing m6A mRNA methylation of IL-17RA[J]. Front Oncol, 2022, 12: 989353. DOI: 10.3389/fonc.2022.989353. |
| [11] |
MITTENBÜHLER MJ, SAEDLER K, NOLTE H, et al. Hepatic FTO is dispensable for the regulation of metabolism but counteracts HCC development in vivo[J]. Mol Metab, 2020, 42: 101085. DOI: 10.1016/j.molmet.2020.101085. |
| [12] |
QIAN XF, ZENG P, LIU JF, et al. Research progress of enzymes related to m 6A RNA methylation modification[J]. Chin J Immunol, 2023, 39( 5): 1073- 1084. DOI: 10.3969/j.issn.1000-484X.2023.05.033. |
| [13] |
LI YC, SU R, DENG XL, et al. FTO in cancer: Functions, molecular mechanisms, and therapeutic implications[J]. Trends Cancer, 2022, 8( 7): 598- 614. DOI: 10.1016/j.trecan.2022.02.010. |
| [14] |
XU ZY, JING X, XIONG XD. Emerging role and mechanism of the FTO gene in cardiovascular diseases[J]. Biomolecules, 2023, 13( 5): 850. DOI: 10.3390/biom13050850. |
| [15] |
WU RF, CHEN YS, LIU YH, et al. m6A methylation promotes white-to-beige fat transition by facilitating Hif1a translation[J]. EMBO Rep, 2021, 22( 11): e52348. DOI: 10.15252/embr.202052348. |
| [16] |
HE Y, YANG WH, GAN LL, et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol, 2021, 44( 5): 355- 365. DOI: 10.1016/j.gastrohep.2020.09.014. |
| [17] |
BEN-HAIM MS, PINTO Y, MOSHITCH-MOSHKOVITZ S, et al. Dynamic regulation of N 6, 2'-O-dimethyladenosine(m 6Am) in obesity[J]. Nat Commun, 2021, 12( 1): 7185. DOI: 10.1038/s41467-021-27421-2. |
| [18] |
YANG Z, YU GL, ZHU X, et al. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: Implications in lipid metabolic disorders[J]. Genes Dis, 2021, 9( 1): 51- 61. DOI: 10.1016/j.gendis.2021.01.005. |
| [19] |
LI Y, YANG F, GAO M, et al. miR-149-3p Regulates the switch between adipogenic and osteogenic differentiation of BMSCs by targeting FTO[J]. Mol Ther Nucleic Acids, 2019, 17: 590- 600. DOI: 10.1016/j.omtn.2019.06.023. |
| [20] |
CHURCH C, MOIR L, MCMURRAY F, et al. Overexpression of FTO leads to increased food intake and results in obesity[J]. Nat Genet, 2010, 42( 12): 1086- 1092. DOI: 10.1038/ng.713. |
| [21] |
LI XC, JIN F, WANG BY, et al. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA[J]. Theranostics, 2019, 9( 13): 3853- 3865. DOI: 10.7150/thno.31868. |
| [22] |
AĞAGÜNDÜZ D, GEZMEN-KARADAĞ M. Association of FTO common variant(rs9939609) with body fat in Turkish individuals[J]. Lipids Health Dis, 2019, 18( 1): 212. DOI: 10.1186/s12944-019-1160-y. |
| [23] |
SUN DL, ZHAO TH, ZHANG Q, et al. Fat mass and obesity-associated protein regulates lipogenesis via m 6A modification in fatty acid synthase mRNA[J]. Cell Biol Int, 2021, 45( 2): 334- 344. DOI: 10.1002/cbin.11490. |
| [24] |
ZHAO LC, FAN TT, HAN YL, et al. Demethylase FTO activity analysis based on methyl sensitive enzyme MazF and hybridization chain reaction[J]. Sens Actuat B Chem, 2021, 341: 129983. DOI: 10.1016/j.snb.2021.129983. |
| [25] |
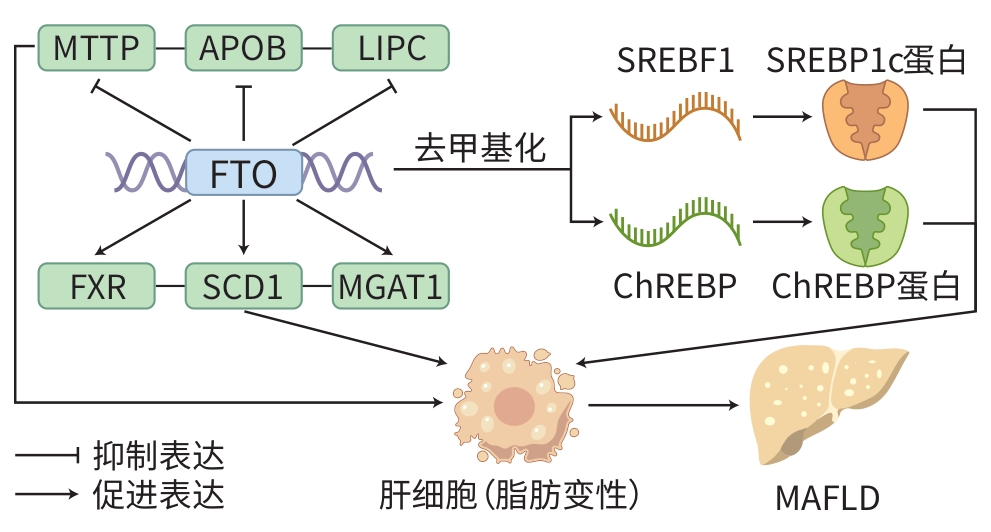
TANG ZL, SUN C, YAN Y, et al. Aberrant elevation of FTO levels promotes liver steatosis by decreasing the m6A methylation and increasing the stability of SREBF1 and ChREBP mRNAs[J]. J Mol Cell Biol, 2023, 14( 9): mjac061. DOI: 10.1093/jmcb/mjac061. |
| [26] |
ZHANG VX, CHEN A, ZHANG QY, et al. FRI-473 The oncogenic m6A demethylase FTO promotes tumorigenesis and immune escape by upregulating GPNMB in hepatocellular carcinoma[J]. J Hepatol, 2024, 80: S419- S420. DOI: 10.1016/S0168-8278(24)01335-7. |
| [27] |
LI R, YAN XJ, XIAO CC, et al. FTO deficiency in older livers exacerbates ferroptosis during ischaemia/reperfusion injury by upregulating ACSL4 and TFRC[J]. Nat Commun, 2024, 15( 1): 4760. DOI: 10.1038/s41467-024-49202-3. |
| [28] |
BIAN XY, SHI DM, XING KL, et al. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation[J]. Clin Transl Med, 2021, 11( 3): e352. DOI: 10.1002/ctm2.352. |
| [29] |
ZHENG JH, WANG FJ, GUO HW, et al. Gut microbiota modulates differential lipid metabolism outcomes associated with FTO gene polymorphisms in response to personalized nutrition intervention[J]. Front Nutr, 2022, 9: 985723. DOI: 10.3389/fnut.2022.985723. |
| [30] |
CHEN XF, GAO Y, YANG XB, et al. Relationship of FTO gene variations with NAFLD risk in Chinese men[J]. Open Life Sci, 2020, 15( 1): 860- 867. DOI: 10.1515/biol-2020-0081. |
| [31] |
GU Z, BI Y, YUAN F, et al. FTO polymorphisms are associated with metabolic dysfunction-associated fatty liver disease(MAFLD) susceptibility in the older Chinese Han population[J]. Clin Interv Aging, 2020, 15: 1333- 1341. DOI: 10.2147/CIA.S254740. |
| [32] |
PANKOVA ED, CHULKOV VS, GAVRILOVA ES, et al. Sequence gene variants in PPARGC1A rs8192678, PPARG2 rs1801282, FTO rs9939609, LEP rs7799039, LEPR rs1137101 and nonalcoholic fatty liver disease[J]. Saratov J Med Sci Res, 2023, 19( 3): 256- 260. DOI: 10.15275/ssmj1903256. |
| [33] |
KANG HF, ZHANG ZW, YU L, et al. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation[J]. J Cell Biochem, 2018, 119( 7): 5676- 5685. DOI: 10.1002/jcb.26746. |
| [34] |
CHANDRASEKARAN P, WEISKIRCHEN R. The role of SCAP/SREBP as central regulators of lipid metabolism in hepatic steatosis[J]. Int J Mol Sci, 2024, 25( 2): 1109. DOI: 10.3390/ijms25021109. |
| [35] |
IIZUKA K, KEN TK, YABE D. ChREBP-mediated regulation of lipid metabolism: Involvement of the gut microbiota, liver, and adipose tissue[J]. Front Endocrinol(Lausanne), 2020, 11: 587189. DOI: 10.3389/fendo.2020.587189. |
| [36] |
DO MH, OH MJ, LEE HB, et al. Bifidobacterium animalis ssp. lactis MG741 reduces body weight and ameliorates nonalcoholic fatty liver disease via improving the gut permeability and amelioration of inflammatory cytokines[J]. Nutrients, 2022, 14( 9): 1965. DOI: 10.3390/nu14091965. |
| [37] |
MANZANO M, GIRON MD, SALTO R, et al. Quality more than quantity: The use of carbohydrates in high-fat diets to tackle obesity in growing rats[J]. Front Nutr, 2022, 9: 809865. DOI: 10.3389/fnut.2022.809865. |
| [38] |
REN Y, HUANG P, ZHANG L, et al. Dual regulation mechanism of obesity: DNA methylation and intestinal flora[J]. Biomedicines, 2024, 12( 8): 1633. DOI: 10.3390/biomedicines12081633. |
| [39] |
ZENG BT, WU RF, CHEN YS, et al. FTO knockout in adipose tissue effectively alleviates hepatic steatosis partially via increasing the secretion of adipocyte-derived IL-6[J]. Gene, 2022, 818: 146224. DOI: 10.1016/j.gene.2022.146224. |
| [40] |
HU Y, FENG Y, ZHANG LC, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m 6A on lipogenic mRNAs[J]. RNA Biol, 2020, 17( 7): 930- 942. DOI: 10.1080/15476286.2020.1736868. |
| [41] |
HEROLD KC, REYNOLDS J, DZIURA J, et al. Exenatide extended release in patients with type 1 diabetes with and without residual insulin production[J]. Diabetes Obes Metab, 2020, 22( 11): 2045- 2054. DOI: 10.1111/dom.14121. |
| [42] |
CHANG Y, DONG MX, WANG Y, et al. GLP-1 gene-modified human umbilical cord mesenchymal stem cell line improves blood glucose level in type 2 diabetic mice[J]. Stem Cells Int, 2019, 2019: 4961865. DOI: 10.1155/2019/4961865. |
| [43] |
LI S, WANG XM, ZHANG JL, et al. Exenatide ameliorates hepatic steatosis and attenuates fat mass and FTO gene expression through PI3K signaling pathway in nonalcoholic fatty liver disease[J]. Braz J Med Biol Res, 2018, 51( 8): e7299. DOI: 10.1590/1414-431x20187299. |
| [44] |
JI FH, FU XH, LI GQ, et al. FTO prevents thyroid cancer progression by SLC7A11 m6A methylation in a ferroptosis-dependent manner[J]. Front Endocrinol(Lausanne), 2022, 13: 857765. DOI: 10.3389/fendo.2022.857765. |
| [45] |
JIANG TY, XIAO Y, ZHOU JF, et al. Arbutin alleviates fatty liver by inhibiting ferroptosis via FTO/SLC7A11 pathway[J]. Redox Biol, 2023, 68: 102963. DOI: 10.1016/j.redox.2023.102963. |
| [46] |
WANG L, FENG YT, WANG JW, et al. Arbutin ameliorates murine colitis by inhibiting JAK2 signaling pathway[J]. Front Pharmacol, 2021, 12: 683818. DOI: 10.3389/fphar.2021.683818. |
| [47] |
PENG SM, XIAO W, JU DP, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1[J]. Sci Transl Med, 2019, 11( 488): eaau7116. DOI: 10.1126/scitranslmed.aau7116. |
| [48] |
VOLLERT J, WANG RS, REGIS S, et al. Genotypes of pain and analgesia in a randomized trial of irritable bowel syndrome[J]. Front Psychiatry, 2022, 13: 842030. DOI: 10.3389/fpsyt.2022.842030. |
| [49] |
FAN CY, HU HT, HUANG XY, et al. Betaine supplementation causes an increase in fatty acid oxidation and carbohydrate metabolism in livers of mice fed a high-fat diet: A proteomic analysis[J]. Foods, 2022, 11( 6): 881. DOI: 10.3390/foods11060881. |
| [50] |
SUN LM, GAO M, QIAN QH, et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m 6A methylation level to cause lipid metabolism disorder in zebrafish[J]. Sci Total Environ, 2021, 770: 145285. DOI: 10.1016/j.scitotenv.2021.145285. |
| [51] |
WANG XX, ZHU LN, CHEN JQ, et al. mRNA m6A methylation downregulates adipogenesis in porcine adipocytes[J]. Biochem Biophys Res Commun, 2015, 459( 2): 201- 207. DOI: 10.1016/j.bbrc.2015.02.048. |
| [52] |
CHENG LD, YU P, LI FF, et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression[J]. Hum Cell, 2021, 34( 6): 1697- 1708. DOI: 10.1007/s13577-021-00593-1. |
| [53] |
MOZES M, GANTSETSEG G, MANZÉGER A, et al.#5503 Pioglitazone reverses miR-130A and miR-199 dysregulation induced by tgf-beta during kidney fibrosis[J]. Nephrol Dial Transplant, 2023, 38( Suppl 1): i476- i477. DOI: 10.1093/ndt/gfad063c_5503. |
| [54] |
AN J, CHENG LJ, YANG LP, et al. P-hydroxybenzyl alcohol alleviates oxidative stress in a nonalcoholic fatty liver disease larval zebrafish model and a BRL-3A hepatocyte via the Nrf2 pathway[J]. Front Pharmacol, 2021, 12: 646239. DOI: 10.3389/fphar.2021.646239. |







 DownLoad:
DownLoad: