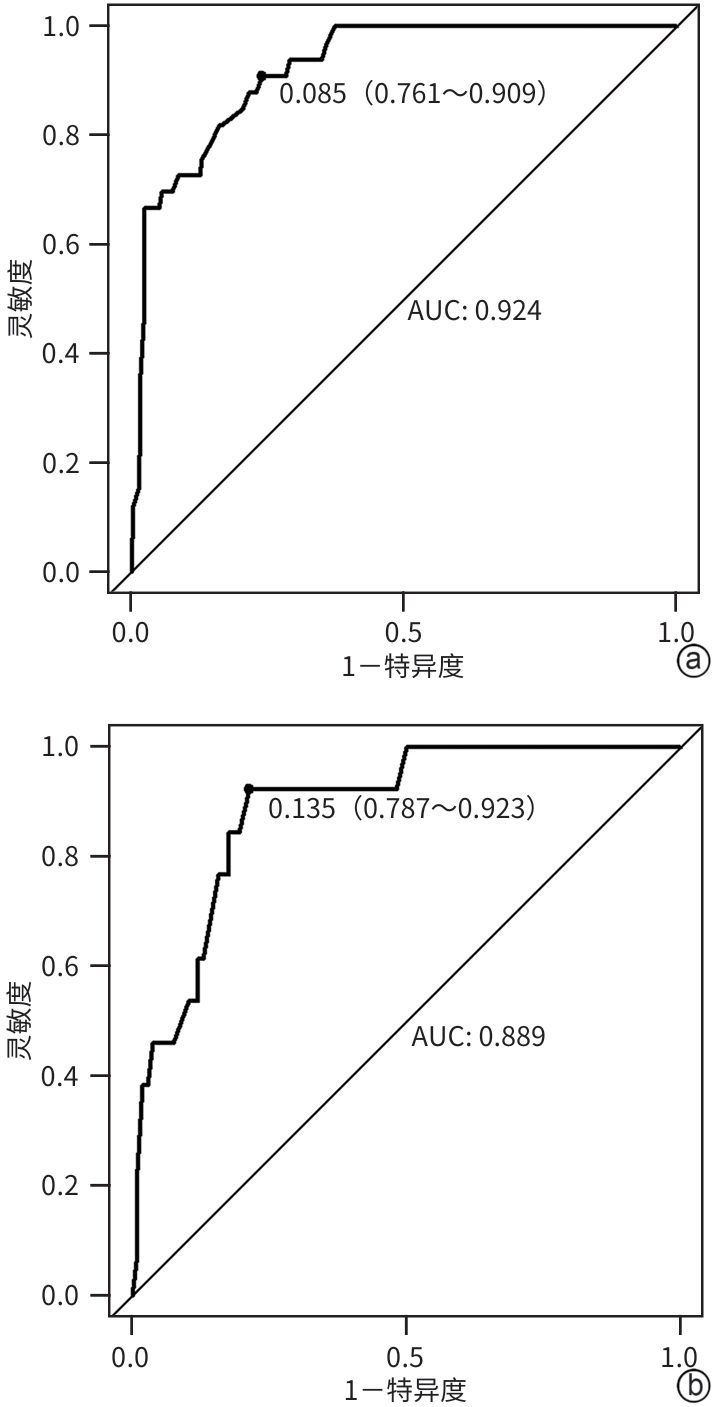
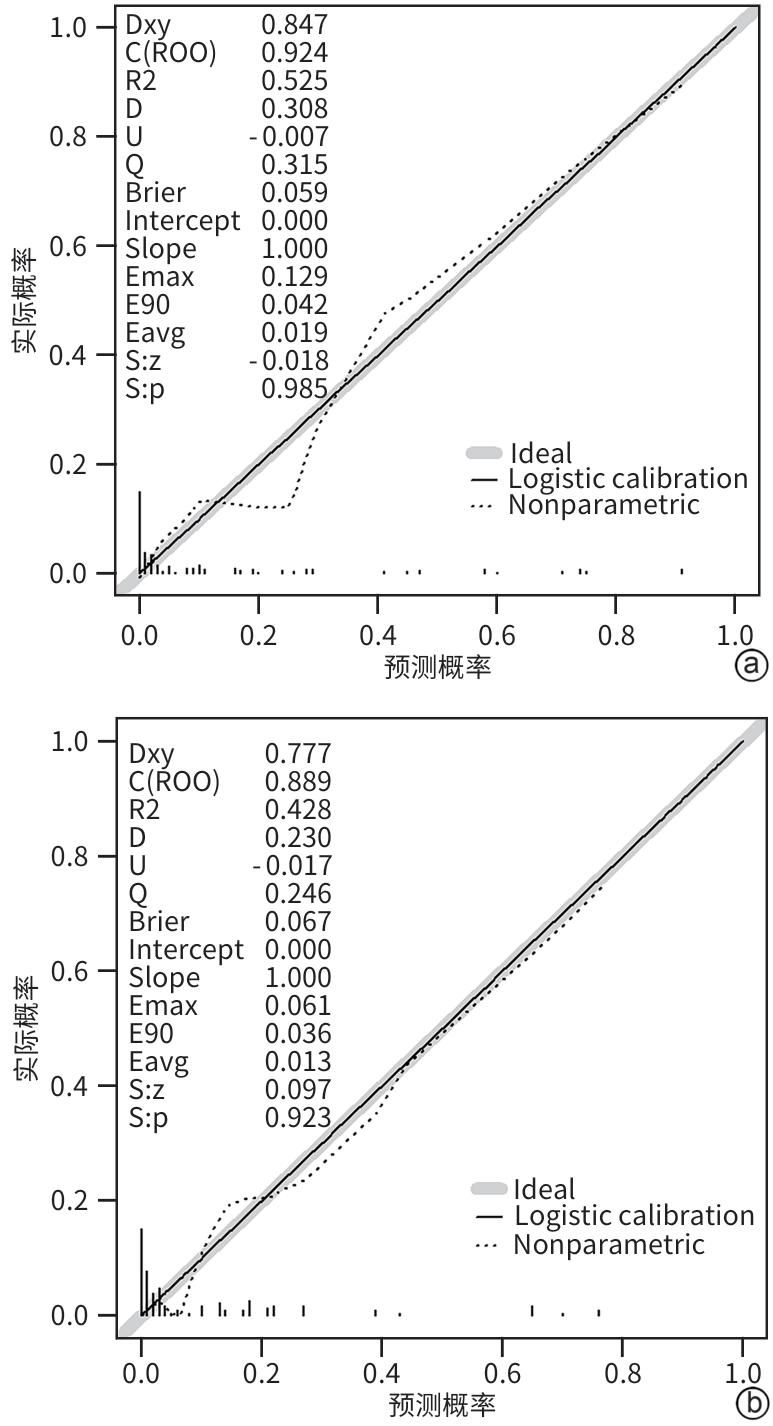
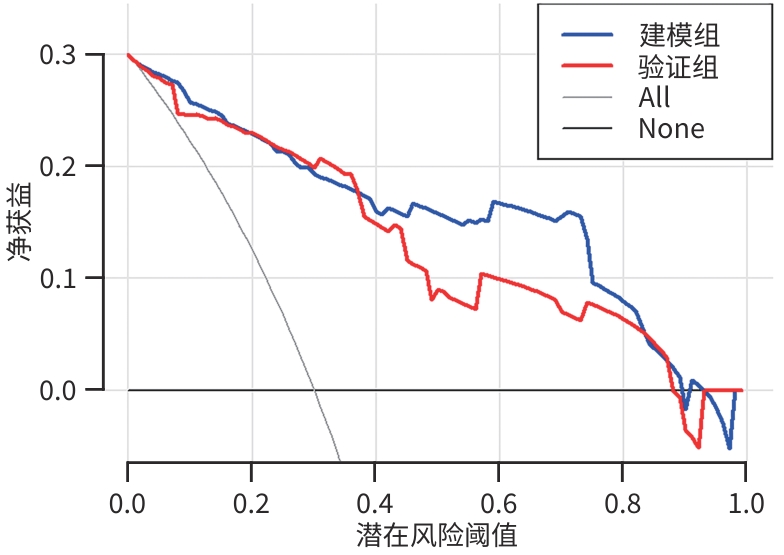
| [1] |
WU SM, ZHOU Q, CAI Y, et al. Development and validation of a prediction model for the early occurrence of acute kidney injury in patients with acute pancreatitis[J]. Ren Fail, 2023, 45( 1): 2194436. DOI: 10.1080/0886022X.2023.2194436. |
| [2] |
|
| [3] |
|
| [4] |
ZHOU HJ, MEI X, HE XH, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study[J]. Medicine(Baltimore), 2019, 98( 16): e15275. DOI: 10.1097/MD.0000000000015275. |
| [5] |
WU ZQ, LIU N, WAN YD, et al. Analysis of risk factors affecting renal function recovery in patients with severe acute pancreatitis[J]. Chin J Emerg Med, 2020, 29( 9): 1173- 1177. DOI: 10.3760/cma.j.issn.1671-0282.2020.09.007. |
| [6] |
YIN JL, ZHAO MM, WANG Y, et al. Analysis of clinical characteristics and influencing factors of disease prognosis in severe acute pancreatitis at different stages[J]. J Clin Exp Med, 2024, 23( 7): 698- 702. DOI: 10.3969/j.issn.1671-4695.2024.07.007. |
| [7] |
|
| [8] |
SILVA-VAZ P, ABRANTES AM, CASTELO-BRANCO M, et al. Multifactorial scores and biomarkers of prognosis of acute pancreatitis: Applications to research and practice[J]. Int J Mol Sci, 2020, 21( 1): 338. DOI: 10.3390/ijms21010338. |
| [9] |
van DEN BERG FF, de BRUIJN AC, van SANTVOORT HC, et al. Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis[J]. Pancreatology, 2020, 20( 7): 1302- 1311. DOI: 10.1016/j.pan.2020.09.007. |
| [10] |
HE J, YU S, ZHANG J. Value of serum interleukin-6 and tumor necrosis factor-α in early diagnosis of severe acute pancreatitis[J]. J Clin Hepatol, 2023, 39( 7): 1657- 1664. DOI: 10.3969/j.issn.1001-5256.2023.07.020. |
| [11] |
SKOURAS C, DAVIS ZA, SHARKEY J, et al. Lung ultrasonography as a direct measure of evolving respiratory dysfunction and disease severity in patients with acute pancreatitis[J]. HPB(Oxford), 2015. DOI: 10.1111/hpb.12515. |
| [12] |
TAYDAS O, UNAL E, KARAOSMANOGLU AD, et al. Accuracy of early CT findings for predicting disease course in patients with acute pancreatitis[J]. Jpn J Radiol, 2018, 36( 2): 151- 158. DOI: 10.1007/s11604-017-0709-9. |
| [13] |
XU WH, LI XH, YU NJ, et al. Comparison of the imaging and clinical characteristics between initial and recurrent alcoholic acute pancreatitis: A retrospective cross-sectional study[J]. Am J Drug Alcohol Abuse, 2023, 49( 4): 431- 439. DOI: 10.1080/00952990.2023.2211221. |
| [14] |
Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of acute pancreatitis in China(2021)[J]. Chin J Dig Surg, 2021, 20( 7): 730- 739. DOI: 10.3760/cma.j.cn115610-20210622-00297. |
| [15] |
ZHANG J, ZHAO W, REN QL, et al. Predictive value of enhanced CT necrotic volume and attenuation value for poor prognosis of acute necrotizing pancreatitis[J]. Mod Dig Interv, 2021, 26( 12): 1584- 1588. DOI: 10.3969/j.issn.1672-2159.2021.12.024. |
| [16] |
MONGODI S, de LUCA D, COLOMBO A, et al. Quantitative lung ultrasound: Technical aspects and clinical applications[J]. Anesthesiology, 2021, 134( 6): 949- 965. DOI: 10.1097/ALN.0000000000003757. |
| [17] |
MORTELE KJ, WIESNER W, INTRIERE L, et al. A modified CT severity index for evaluating acute pancreatitis: Improved correlation with patient outcome[J]. AJR Am J Roentgenol, 2004, 183( 5): 1261- 1265. DOI: 10.2214/ajr.183.5.1831261. |
| [18] |
de WAELE JJ, DELRUE L, HOSTE EA, et al. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: Evaluation of a new scoring system[J]. Pancreas, 2007, 34( 2): 185- 190. DOI: 10.1097/mpa.0b013e31802d4136. |
| [19] |
NAWACKI Ł, GŁUSZEK S. Hospital mortality rate and predictors in acute pancreatitis in Poland: A single-center experience[J]. Asian J Surg, 2024, 47( 1): 208- 215. DOI: 10.1016/j.asjsur.2023.07.063. |
| [20] |
HIDALGO NJ, PANDO E, MATA R, et al. Impact of comorbidities on hospital mortality in patients with acute pancreatitis: A population-based study of 110, 021 patients[J]. BMC Gastroenterol, 2023, 23( 1): 81. DOI: 10.1186/s12876-023-02730-6. |
| [21] |
LEPPÄNIEMI A, TOLONEN M, TARASCONI A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis[J]. World J Emerg Surg, 2019, 14: 27. DOI: 10.1186/s13017-019-0247-0. |
| [22] |
AN WH, HE XC, YANG J, et al. Value of early admission scoring systems in predicting the severity and prognosis of acute pancreatitis[J]. J Clin Hepatol, 2020, 36( 6): 1342- 1346. DOI: 10.3969/j.issn.1001-5256.2020.06.030. |
| [23] |
PALIWAL A, NAWAL CL, MEENA PD, et al. A study of procalcitonin as an early predictor of severity in acute pancreatitis[J]. J Assoc Physicians India, 2022, 70( 4): 11- 12.
|
| [24] |
MATHAI MJ, REDDY M VS, SHETTY V. Analysis of the accuracy of the modified CT severity index in predicting clinical outcomes in acute pancreatitis: A cross-sectional study[J]. Cureus, 2024, 16( 3): e56123. DOI: 10.7759/cureus.56123. |
| [25] |
HU XY, YANG ZY, ZHAO CJ, et al. Research progress in acute pancreatitis scoring systems in predicting the severity of disease[J/OL]. Chin J Hepat Surg(Electronic Edition), 2024, 13( 2): 239- 243. DOI: 10.3877/cma.j.issn.2095-3232.2024.02.021. |
| [26] |
STERNBY H, HARTMAN H, JOHANSEN D, et al. IL-6 and CRP are superior in early differentiation between mild and non-mild acute pancreatitis[J]. Pancreatology, 2017, 17( 4): 550- 554. DOI: 10.1016/j.pan.2017.05.392. |
| [27] |
SUN JX, YAN X. Improved CT severity index combined with IL-6 to predict hospitalization death of acute pancreatitis[J]. Mod Interv Diagn Treat Gastroenterol, 2023, 28( 2): 244- 248. DOI: 10.3969/j.issn.1672-2159.2023.02.023. |
| [28] |
LI P, JIAN JN, CHEN RL. Effect of early enteral nutrition on serum inflammatory factors and intestinal mucosal permeability in patients with severe acute pancreatitis[J]. Turk J Gastroenterol, 2021, 32( 10): 907- 912. DOI: 10.5152/tjg.2021.201033. |
| [29] |
CARDOSO FS, RICARDO LB, OLIVEIRA AM, et al. C-reactive protein prognostic accuracy in acute pancreatitis: Timing of measurement and cutoff points[J]. Eur J Gastroenterol Hepatol, 2013, 25( 7): 784- 789. DOI: 10.1097/MEG.0b013e32835fd3f0. |
| [30] |
SAAD H, ERAKY M, EL-TAHE A, et al. A thorough study and meta-analysis of the prognostic relevance of the c-reactive-albumin ratio in acute pancreatitis[J]. Georgian Med News, 2023,( 343): 111- 118.
|
| [31] |
SATHYANARAYAN G, GARG PK, PRASAD H, et al. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis[J]. J Gastroenterol Hepatol, 2007, 22( 4): 550- 554. DOI: 10.1111/j.1440-1746.2006.04752.x. |
| [32] |
HUANG ZY, MA X, JIA XT, et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: A randomized controlled clinical trial[J]. Am J Gastroenterol, 2020, 115( 3): 473- 480. DOI: 10.14309/ajg.0000000000000529. |
| [33] |
HAN BH, YANG W, WANG H, et al. Construction and evaluation of a prognostic model for severe acute pancreatitis based on CT scores and inflammatory factors[J]. Chin Crit Care Med, 2023, 35( 1): 82- 87. DOI: 10.3760/cma.j.cn121430-20220411-00351. |
| [34] |
SAHU B, ABBEY P, ANAND R, et al. Severity assessment of acute pancreatitis using CT severity index and modified CT severity index: Correlation with clinical outcomes and severity grading as per the Revised Atlanta Classification[J]. Indian J Radiol Imaging, 2017, 27( 2): 152- 160. DOI: 10.4103/ijri.IJRI_300_16. |
| [35] |
GEZER NS, BENGI G, BARAN A, et al. Comparison of radiological scoring systems, clinical scores, neutrophil-lymphocyte ratio and serum C-reactive protein level for severity and mortality in acute pancreatitis[J]. Rev Assoc Med Bras(1992), 2020, 66( 6): 762- 770. DOI: 10.1590/1806-9282.66.6.762. |
| [36] |
ZHAO Z, JIANG L, XI XM, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome[J]. BMC Pulm Med, 2015, 15: 98. DOI: 10.1186/s12890-015-0091-2. |
| [37] |
YANG Q, LUO YL, LAN BW, et al. Fighting fire with fire: Exosomes and acute pancreatitis-associated acute lung injury[J]. Bioengineering(Basel), 2022, 9( 11): 615. DOI: 10.3390/bioengineering9110615. |
| [38] |
WANG XY, WANG ZP, WU J, et al. Effect of early thoracic paracentesis drainage on acute lung injury in severe acute pancreatitis[J]. J Clin Hepatol, 2023, 39( 7): 1633- 1642. DOI: 10.3969/j.issn.1001-5256.2023.07.018. |
| [39] |
RANA SS, KATAGERI B, SHAH J, et al. Role of transthoracic lung ultrasonography in acute pancreatitis[J]. Pancreas, 2020, 49( 5): e47- e48. DOI: 10.1097/MPA.0000000000001556. |



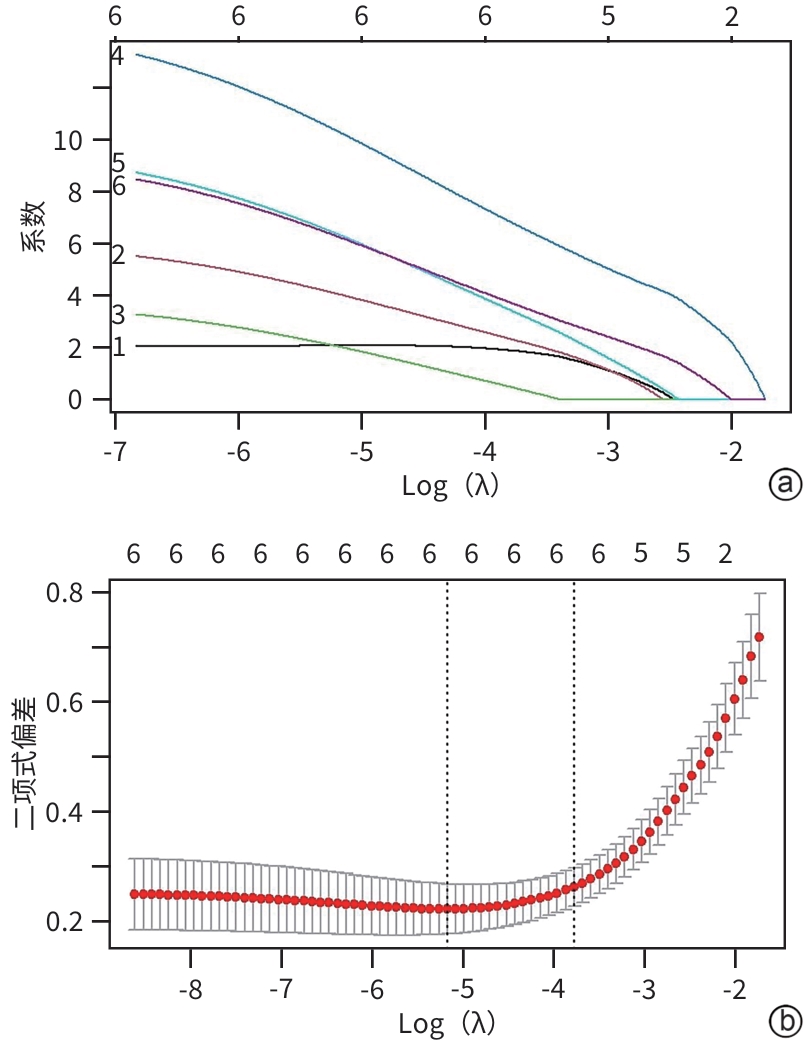




 DownLoad:
DownLoad: