| [1] |
TARGHER G, BYRNE CD, TILG H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications[J]. Gut, 2024, 73( 4): 691- 702. DOI: 10.1136/gutjnl-2023-330595. |
| [2] |
YOUNOSSI Z, TACKE F, ARRESE M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69( 6): 2672- 2682. DOI: 10.1002/hep.30251. |
| [3] |
PETTA S, TARGHER G, ROMEO S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom[J]. Liver Int, 2024, 44( 7): 1526- 1536. DOI: 10.1111/liv.15930. |
| [4] |
RINELLA ME, NEUSCHWANDER-TETRI BA, SIDDIQUI MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease[J]. Hepatology, 2023, 77( 5): 1797- 1835. DOI: 10.1097/HEP.0000000000000323. |
| [5] |
SHI L, JIN L, HUANG W. Bile acids, intestinal barrier dysfunction, and related diseases[J]. Cells, 2023, 12( 14): 1888. DOI: 10.3390/cells12141888. |
| [6] |
WANG JJ, CAI XB, LU LG. Association between bile acids and nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 5): 1166- 1171. DOI: 10.3969/j.issn.1001-5256.2023.05.026. |
| [7] |
CHÁVEZ-TALAVERA O, HAAS J, GRZYCH G, et al. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: What do the human studies tell?[J]. Curr Opin Lipidol, 2019, 30( 3): 244- 254. DOI: 10.1097/MOL.0000000000000597. |
| [8] |
POUPON R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: An overview of their mechanisms of action[J]. Clin Res Hepatol Gastroenterol, 2012, 36( Suppl 1): S3- S12. DOI: 10.1016/S2210-7401(12)70015-3. |
| [9] |
ALKHOURI N, LACERTE C, EDWARDS J, et al. Safety, pharmacokinetics and pharmacodynamics of obeticholic acid in subjects with fibrosis or cirrhosis from NASH[J]. Liver Int, 2024, 44( 4): 966- 978. DOI: 10.1111/liv.15816. |
| [10] |
DENK H, ABUJA PM, ZATLOUKAL K. Animal models of NAFLD from the pathologist’s point of view[J]. Biochim Biophys Acta BBA Mol Basis Dis, 2019, 1865( 5): 929- 942. DOI: 10.1016/j.bbadis.2018.04.024. |
| [11] |
TSUCHIDA T, LEE YA, FUJIWARA N, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer[J]. J Hepatol, 2018, 69( 2): 385- 395. DOI: 10.1016/j.jhep.2018.03.011. |
| [12] |
TSOUKA S, KUMAR P, SEUBNOOCH P, et al. Transcriptomics-driven metabolic pathway analysis reveals similar alterations in lipid metabolism in mouse MASH model and human[J]. Commun Med(Lond), 2024, 4( 1): 39. DOI: 10.1038/s43856-024-00465-3. |
| [13] |
ZHANG LZ, SHI JW, SHEN Q, et al. Astragalus saponins protect against extrahepatic and intrahepatic cholestatic liver fibrosis models by activation of farnesoid X receptor[J]. J Ethnopharmacol, 2024, 318( Pt A): 116833. DOI: 10.1016/j.jep.2023.116833. |
| [14] |
ZHANG LZ, JIANG XY, SHI JW, et al. Isoastragaloside I attenuates cholestatic liver diseases by ameliorating liver injury, regulating bile acid metabolism and restoring intestinal barrier[J]. J Ethnopharmacol, 2024, 335: 118649. DOI: 10.1016/j.jep.2024.118649. |
| [15] |
European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64( 6): 1388- 1402. DOI: 10.1016/j.jhep.2015.11.004. |
| [16] |
Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of metabolic dysfunction-associated(non-alcoholic)fatty liver disease(Version 2024)[J]. J Pract Hepatol, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163. |
| [17] |
ANGULO P, KLEINER DE, DAM-LARSEN S, et al. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2015, 149( 2): 389- 397. e 10. DOI: 10.1053/j.gastro.2015.04.043. |
| [18] |
KLEINER DE, MAKHLOUF HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children[J]. Clin Liver Dis, 2016, 20( 2): 293- 312. DOI: 10.1016/j.cld.2015.10.011. |
| [19] |
BERGER D, DESAI V, JANARDHAN S. Con: Liver biopsy remains the gold standard to evaluate fibrosis in patients with nonalcoholic fatty liver disease[J]. Clin Liver Dis(Hoboken), 2019, 13( 4): 114- 116. DOI: 10.1002/cld.740. |
| [20] |
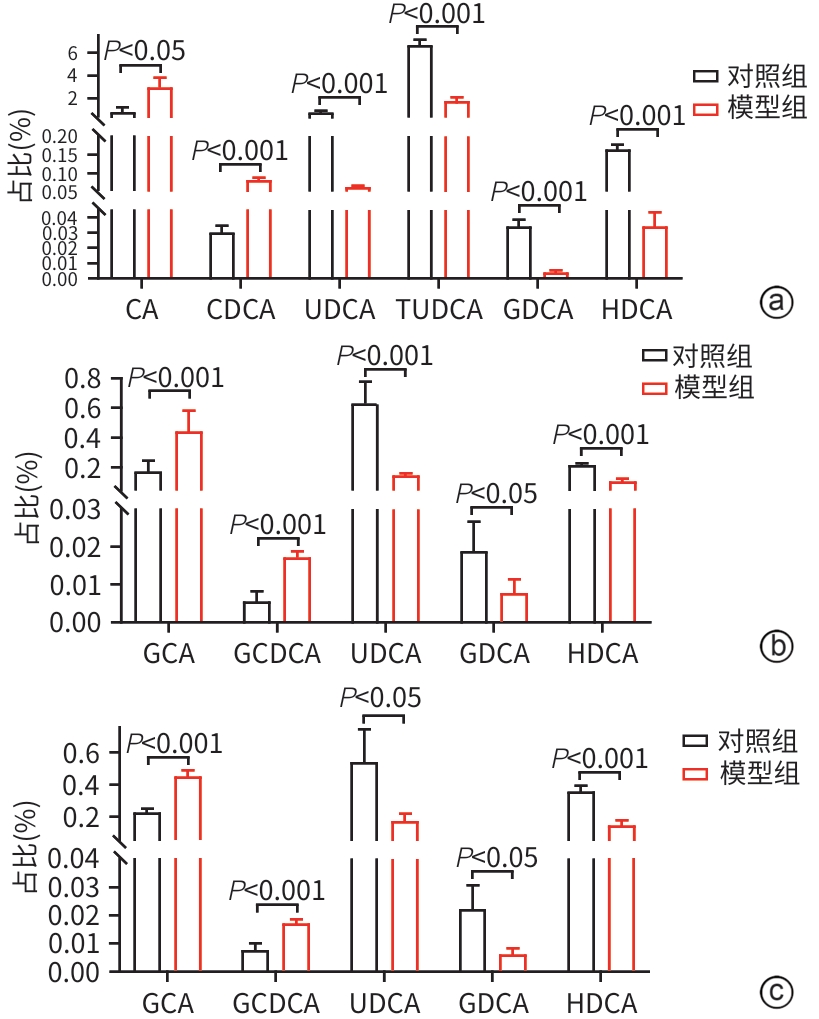
YANG HL, ZHANG DQ, CHEN GF, et al. Changes of serum and liver bile acid profiles in a mouse model of metabolic associated fatty liver disease induced by a methionine-choline-deficient diet[J]. Chin J Tissue Eng Res, 2021, 25( 26): 4137- 4144.
杨海琳, 张定棋, 陈高峰, 等. 蛋氨酸-胆碱缺乏饮食诱导代谢相关脂肪肝模型小鼠血清和肝脏胆汁酸谱的变化[J]. 中国组织工程研究, 2021, 25( 26): 4137- 4144.
|
| [21] |
BRUNT EM, KLEINER DE, WILSON LA, et al. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: Results from the nonalcoholic steatohepatitis clinical research network treatment trials[J]. Hepatology, 2019, 70( 2): 522- 531. DOI: 10.1002/hep.30418. |
| [22] |
KANG JH, LI MJ, LUAN PP, et al. Establishment of non-alcoholic steatohepatitis mouse model induced by Western diet combined with low-dose carbon tetrachloride[J]. J Shanghai Jiao Tong Univ Med Sci, 2020, 40( 5): 590- 597. DOI: 10.3969/j.issn.1674-8115.2020.05.005. |
| [23] |
BASUNI AA, SWEED D, ELGAZZAR MF, et al. Exploring serum bile acids as potential noninvasive biomarkers for nonalcoholic fatty liver disease[J]. Egypt Liver J, 2024, 14( 1): 70. DOI: 10.1186/s43066-024-00378-9. |
| [24] |
FENG S, XIE XM, LI JC, et al. Bile acids induce liver fibrosis through the NLRP3 inflammasome pathway and the mechanism of FXR inhibition of NLRP3 activation[J]. Hepatol Int, 2024, 18( 3): 1040- 1052. DOI: 10.1007/s12072-023-10610-0. |
| [25] |
LIN XZ, MAI MQ, HE TP, et al. Efficiency of ursodeoxycholic acid for the treatment of nonalcoholic steatohepatitis: A systematic review and meta-analysis[J]. Expert Rev Gastroenterol Hepatol, 2022, 16( 6): 537- 545. DOI: 10.1080/17474124.2022.2083605. |
| [26] |
KUANG JL, WANG JY, LI YT, et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis[J]. Cell Metab, 2023, 35( 10): 1752- 1766. e 8. DOI: 10.1016/j.cmet.2023.07.011. |
| [27] |
NIE QX, LUO X, WANG K, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway[J]. Cell, 2024, 187( 11): 2717- 2734. e 33. DOI: 10.1016/j.cell.2024.03.034. |
| [28] |
ZHENG LT, WANG Z, CHEN YC, et al. Role of Akkermansia muciniphila in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2024, 40( 3): 594- 599. DOI: 10.12449/JCH240326. 郑立婷, 汪哲, 陈玉春, 等. 嗜黏蛋白阿克曼菌在非酒精性脂肪性肝病中的作用[J]. 临床肝胆病杂志, 2024, 40( 3): 594- 599. DOI: 10.12449/JCH240326. |



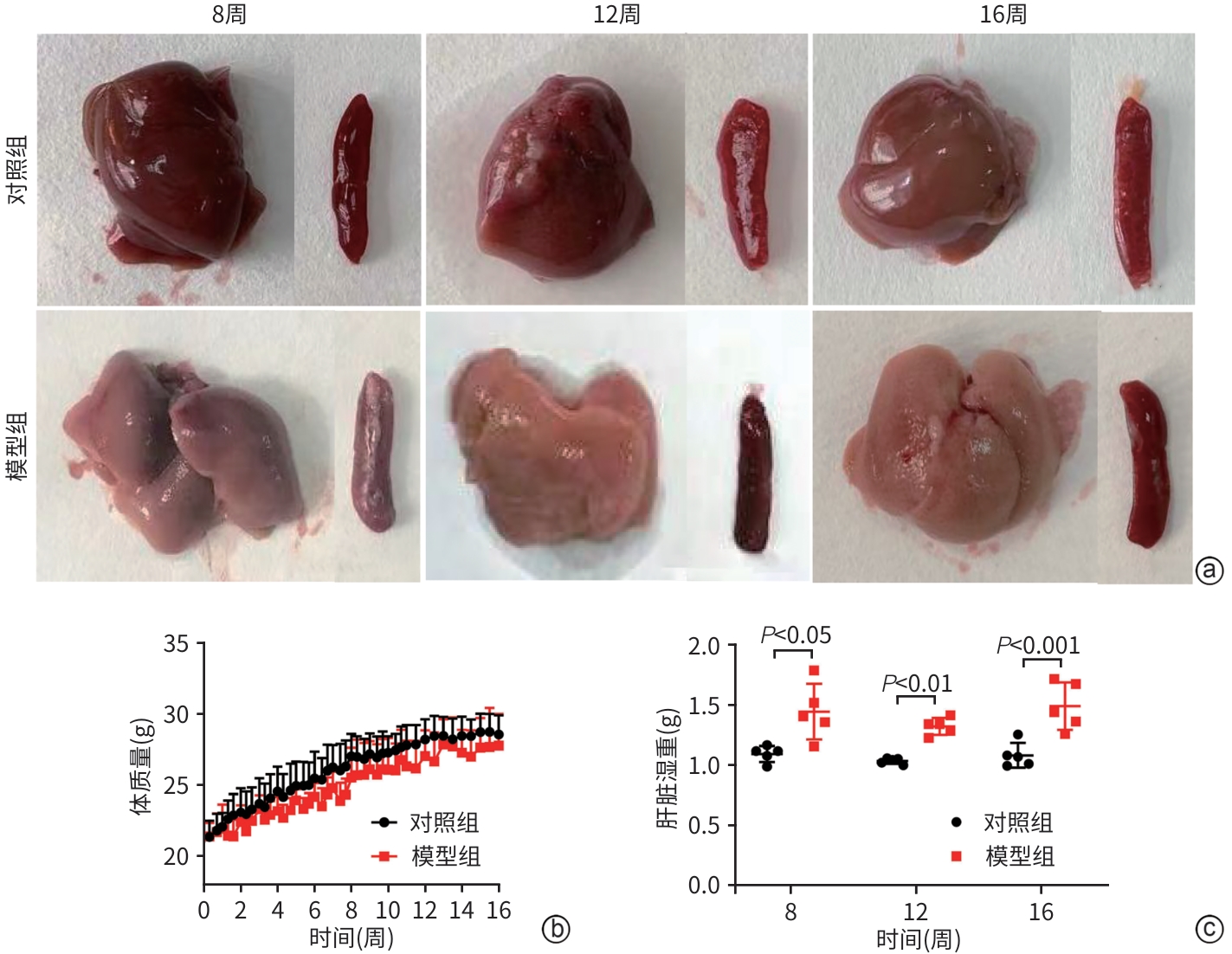




 DownLoad:
DownLoad: