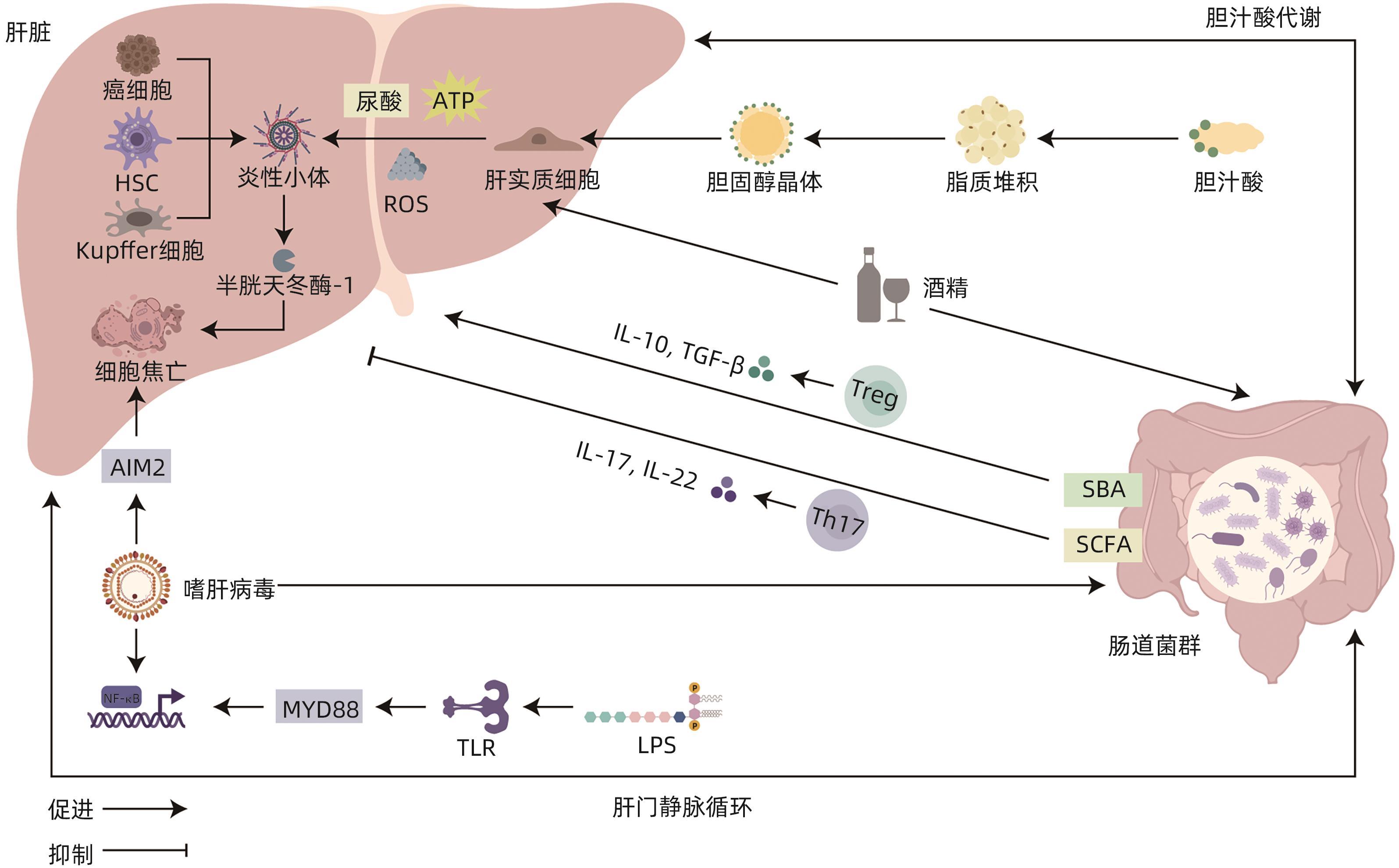
| [1] |
de VOS WM, TILG H, van HUL M, et al. Gut microbiome and health: Mechanistic insights[J]. Gut, 2022, 71( 5): 1020- 1032. DOI: 10.1136/gutjnl-2021-326789. |
| [2] |
PABST O, HORNEF MW, SCHAAP FG, et al. Gut-liver axis: Barriers and functional circuits[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 7): 447- 461. DOI: 10.1038/s41575-023-00771-6. |
| [3] |
KNORR J, WREE A, FELDSTEIN AE. Pyroptosis in steatohepatitis and liver diseases[J]. J Mol Biol, 2022, 434( 4): 167271. DOI: 10.1016/j.jmb.2021.167271. |
| [4] |
GIUFFRÈ M, CAMPIGOTTO M, CAMPISCIANO G, et al. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature[J]. Am J Physiol Gastrointest Liver Physiol, 2020, 318( 5): G889- G906. DOI: 10.1152/ajpgi.00161.2019. |
| [5] |
KNORR J, WREE A, FELDSTEIN AE. Pyroptosis in steatohepatitis and liver diseases[J] Mol Biol, 2022, 434( 4): 167271. DOI: 10.1016/j.jmb.2021.167271. |
| [6] |
CHOPYK DM, GRAKOUI A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159( 3): 849- 863. DOI: 10.1053/j.gastro.2020.04.077. |
| [7] |
ECKBURG PB, BIK EM, BERNSTEIN CN, et al. Diversity of the human intestinal microbial flora[J]. Science, 2005, 308( 5728): 1635- 1638. DOI: 10.1126/science.1110591. |
| [8] |
MOWAT AM, AGACE WW. Regional specialization within the intestinal immune system[J]. Nat Rev Immunol, 2014, 14( 10): 667- 685. DOI: 10.1038/nri3738. |
| [9] |
BEL S, PENDSE M, WANG YH, et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine[J]. Science, 2017, 357( 6355): 1047- 1052. DOI: 10.1126/science.aal4677. |
| [10] |
MÖRBE UM, JØRGENSEN PB, FENTON TM, et al. Human gut-associated lymphoid tissues(GALT); diversity, structure, and function[J]. Mucosal Immunol, 2021, 14( 4): 793- 802. DOI: 10.1038/s41385-021-00389-4. |
| [11] |
YANG WJ, CONG YZ. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases[J]. Cell Mol Immunol, 2021, 18( 4): 866- 877. DOI: 10.1038/s41423-021-00661-4. |
| [12] |
ANDERSON MJ, DEN HARTIGH AB, FINK SL. Molecular mechanisms of pyroptosis[J]. Methods Mol Biol, 2023, 2641: 1- 16. DOI: 10.1007/978-1-0716-3040-2_1. |
| [13] |
SANTOS JC, DICK MS, LAGRANGE B, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation[J]. EMBO J, 2018, 37( 6): e98089. DOI: 10.15252/embj.201798089. |
| [14] |
WANG JF, DONG R, ZHENG S. Roles of the inflammasome in the gut-liver axis(Review)[J]. Mol Med Rep, 2019, 19( 1): 3- 14. DOI: 10.3892/mmr.2018.9679. |
| [15] |
FERNANDES-ALNEMRI T, WU J, YU JW, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation[J]. Cell Death Differ, 2007, 14( 9): 1590- 1604. DOI: 10.1038/sj.cdd.4402194. |
| [16] |
|
| [17] |
CHEN KW, DEMARCO B, BROZ P. Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation[J]. Eur J Immunol, 2020, 50( 2): 170- 177. DOI: 10.1002/eji.201948254. |
| [18] |
HUANG XL, FENG Y, XIONG GQ, et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis[J]. Cell Biosci, 2019, 9: 31. DOI: 10.1186/s13578-019-0292-0. |
| [19] |
WANG YP, GAO WQ, SHI XY, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547( 7661): 99- 103. DOI: 10.1038/nature22393. |
| [20] |
ZHOU ZW, HE HB, WANG K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells[J]. Science, 2020, 368( 6494): eaaz7548. DOI: 10.1126/science.aaz7548. |
| [21] |
BARNETT KC, LI SR, LIANG KX, et al. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases[J]. Cell, 2023, 186( 11): 2288- 2312. DOI: 10.1016/j.cell.2023.04.025. |
| [22] |
ZHANG YW, YANG WL, LI WG, et al. NLRP3 inflammasome: Checkpoint connecting innate and adaptive immunity in autoimmune diseases[J]. Front Immunol, 2021, 12: 732933. DOI: 10.3389/fimmu.2021.732933. |
| [23] |
MARTENS EC, NEUMANN M, DESAI MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier[J]. Nat Rev Microbiol, 2018, 16( 8): 457- 470. DOI: 10.1038/s41579-018-0036-x. |
| [24] |
XUE SG, XUE Y, DOU DB, et al. Kui Jie Tong ameliorates ulcerative colitis by regulating gut microbiota and NLRP3/caspase-1 classical pyroptosis signaling pathway[J]. Dis Markers, 2022, 2022: 2782112. DOI: 10.1155/2022/2782112. |
| [25] |
|
| [26] |
HAO HP, CAO LJ, JIANG CT, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis[J]. Cell Metab, 2017, 25( 4): 856- 867.e5. DOI: 10.1016/j.cmet.2017.03.007. |
| [27] |
|
| [28] |
HAN CY, RHO HS, KIM A, et al. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury[J]. Cell Rep, 2018, 24( 11): 2985- 2999. DOI: 10.1016/j.celrep.2018.07.068. |
| [29] |
CHEN Y, LE TH, DU QM, et al. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling[J]. Int Immunopharmacol, 2019, 71: 144- 154. DOI: 10.1016/j.intimp.2019.01.021. |
| [30] |
HANG SY, PAIK D, YAO LN, et al. Bile acid metabolites control T H17 and T reg cell differentiation[J]. Nature, 2019, 576( 7785): 143- 148. DOI: 10.1038/s41586-019-1785-z. |
| [31] |
HUANG FJ, ZHENG XJ, MA XH, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism[J]. Nat Commun, 2019, 10( 1): 4971. DOI: 10.1038/s41467-019-12896-x. |
| [32] |
MANNS MP, LOHSE AW, VERGANI D. Autoimmune hepatitis-update 2015[J]. J Hepatol, 2015, 62( 1): S100- S111. DOI: 10.1016/j.jhep.2015.03.005. |
| [33] |
LIU QY, HE W, TANG RQ, et al. Intestinal homeostasis in autoimmune liver diseases[J]. Chin Med J, 2022, 135( 14): 1642- 1652. DOI: 10.1097/CM9.0000000000002291. |
| [34] |
LI LP, KANG YB. The gut microbiome and autoimmune hepatitis: Implications for early diagnostic biomarkers and novel therapies[J]. Mol Nutr Food Res, 2023, 67( 24): e2300043. DOI: 10.1002/mnfr.202300043. |
| [35] |
CHEN JN, WEI YF, HE JQ, et al. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota[J]. Sci Rep, 2014, 4: 7259. DOI: 10.1038/srep07259. |
| [36] |
KANG YB, KUANG XY, YAN H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway[J]. mSystems, 2023, 8( 2): e0112722. DOI: 10.1128/msystems.01127-22. |
| [37] |
WANG H, WANG GD, LIANG YJ, et al. Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity[J]. Free Radic Biol Med, 2019, 143: 223- 231. DOI: 10.1016/j.freeradbiomed.2019.08.014. |
| [38] |
SEITZ HK, BATALLER R, CORTEZ-PINTO H, et al. Alcoholic liver disease[J]. Nat Rev Dis Primers, 2018, 4( 1): 16. DOI: 10.1038/s41572-018-0014-7. |
| [39] |
CHO YE, SONG BJ. Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress[J]. Redox Biol, 2018, 18: 266- 278. DOI: 10.1016/j.redox.2018.07.012. |
| [40] |
PETRASEK J, IRACHETA-VELLVE A, SAHA B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease[J]. J Leukoc Biol, 2015, 98( 2): 249- 256. DOI: 10.1189/jlb.3AB1214-590R. |
| [41] |
WU S, WEN F, ZHONG XB, et al. Astragaloside IV ameliorate acute alcohol-induced liver injury in mice via modulating gut microbiota and regulating NLRP3/caspase-1 signaling pathway[J]. Ann Med, 2023, 55( 1): 2216942. DOI: 10.1080/07853890.2023.2216942. |
| [42] |
KHANOVA E, WU R, WANG W, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients[J]. Hepatology, 2018, 67( 5): 1737- 1753. DOI: 10.1002/hep.29645. |
| [43] |
CALIGIURI A, GENTILINI A, MARRA F. Molecular pathogenesis of NASH[J]. Int J Mol Sci, 2016, 17( 9): 1575. DOI: 10.3390/ijms17091575. |
| [44] |
MUSSO G, CASSADER M, GAMBINO R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies[J]. Nat Rev Drug Discov, 2016, 15( 4): 249- 274. DOI: 10.1038/nrd.2015.3. |
| [45] |
BASHIARDES S, SHAPIRO H, ROZIN S, et al. Non-alcoholic fatty liver and the gut microbiota[J]. Mol Metab, 2016, 5( 9): 782- 794. DOI: 10.1016/j.molmet.2016.06.003. |
| [46] |
TIAN GG, WANG W, XIA ER, et al. Dendrobium officinale alleviates high-fat diet-induced nonalcoholic steatohepatitis by modulating gut microbiota[J]. Front Cell Infect Microbiol, 2023, 13: 1078447. DOI: 10.3389/fcimb.2023.1078447. |
| [47] |
CSAK T, GANZ M, PESPISA J, et al. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells[J]. Hepatology, 2011, 54( 1): 133- 144. DOI: 10.1002/hep.24341. |
| [48] |
ZHU YW, ZHAO H, LU J, et al. Caspase-11-mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis[J]. Cell Mol Gastroenterol Hepatol, 2021, 12( 2): 653- 664. DOI: 10.1016/j.jcmgh.2021.04.009. |
| [49] |
YANG JJ, WANG DW, LI YC, et al. Metabolomics in viral hepatitis: Advances and review[J]. Front Cell Infect Microbiol, 2023, 13: 1189417. DOI: 10.3389/fcimb.2023.1189417. |
| [50] |
SEHGAL R, BEDI O, TREHANPATI N. Role of microbiota in pathogenesis and management of viral hepatitis[J]. Front Cell Infect Microbiol, 2020, 10: 341. DOI: 10.3389/fcimb.2020.00341. |
| [51] |
LU YX, HE CZ, WANG YX, et al. Effect of entecavir on the intestinal microflora in patients with chronic hepatitis B: A controlled cross-sectional and longitudinal real-world study[J]. Infect Dis Ther, 2021, 10( 1): 241- 252. DOI: 10.1007/s40121-020-00355-w. |
| [52] |
INOUE T, NAKAYAMA J, MORIYA K, et al. Gut dysbiosis associated with hepatitis C virus infection[J]. Clin Infect Dis, 2018, 67( 6): 869- 877. DOI: 10.1093/cid/ciy205. |
| [53] |
NEGASH AA, RAMOS HJ, CROCHET N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease[J]. PLoS Pathog, 2013, 9( 4): e1003330. DOI: 10.1371/journal.ppat.1003330. |
| [54] |
YU X, LAN PX, HOU XB, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production[J]. J Hepatol, 2017, 66( 4): 693- 702. DOI: 10.1016/j.jhep.2016.12.018. |
| [55] |
XIE WH, DING J, XIE XX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation[J]. Inflamm Res, 2020, 69( 7): 683- 696. DOI: 10.1007/s00011-020-01351-z. |
| [56] |
PAN XF, XU HX, ZHENG CL, et al. Human hepatocytes express absent in melanoma 2 and respond to hepatitis B virus with interleukin-18 expression[J]. Virus Genes, 2016, 52( 4): 445- 452. DOI: 10.1007/s11262-016-1327-9. |
| [57] |
HAN Y, CHEN Z, HOU R, et al. Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients[J]. Virol J, 2015, 12: 129. DOI: 10.1186/s12985-015-0360-y. |
| [58] |
HO DWH, LO RCL, CHAN LK, et al. Molecular pathogenesis of hepatocellular carcinoma[J]. Liver Cancer, 2016, 5( 4): 290- 302. DOI: 10.1159/000449340. |
| [59] |
LUO WY, GUO SQ, ZHOU Y, et al. Hepatocellular carcinoma: How the gut microbiota contributes to pathogenesis, diagnosis, and therapy[J]. Front Microbiol, 2022, 13: 873160. DOI: 10.3389/fmicb.2022.873160. |
| [60] |
BI CC, XIAO GQ, LIU CY, et al. Molecular immune mechanism of intestinal microbiota and their metabolites in the occurrence and development of liver cancer[J]. Front Cell Dev Biol, 2021, 9: 702414. DOI: 10.3389/fcell.2021.702414. |
| [61] |
ZHAO HJ, ZHANG YM, ZHANG YT, et al. The role of NLRP3 inflammasome in hepatocellular carcinoma[J]. Front Pharmacol, 2023, 14: 1150325. DOI: 10.3389/fphar.2023.1150325. |
| [62] |
LIU BY, ZHOU ZW, JIN Y, et al. Hepatic stellate cell activation and senescence induced by intrahepatic microbiota disturbances drive progression of liver cirrhosis toward hepatocellular carcinoma[J]. J Immunother Cancer, 2022, 10( 1): e003069. DOI: 10.1136/jitc-2021-003069. |
| [63] |
CHEN WB, DING M, JI LY, et al. Bile acids promote the development of HCC by activating inflammasome[J]. Hepatol Commun, 2023, 7( 9): e0217. DOI: 10.1097/HC9.0000000000000217. |
| [64] |
WEI Q, GUO PB, MU K, et al. Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome[J]. Lab Invest, 2015, 95( 7): 804- 816. DOI: 10.1038/labinvest.2015.63. |
| [65] |
ZHANG Y, YANG H, SUN MF, et al. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis[J]. Pharmacol Rep, 2020, 72( 5): 1370- 1382. DOI: 10.1007/s43440-020-00064-8. |
| [66] |
BHAT AA, THAPA R, AFZAL O, et al. The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review[J]. Int J Biol Macromol, 2023, 242( Pt 2): 124832. DOI: 10.1016/j.ijbiomac.2023.124832. |
| [67] |
SHANGGUAN FG, ZHOU HF, MA NF, et al. A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis[J]. Front Cell Dev Biol, 2021, 9: 697832. DOI: 10.3389/fcell.2021.697832. |








 DownLoad:
DownLoad: