| [1] |
STÖLZEL U, DOSS MO, SCHUPPAN D. Clinical guide and update on porphyrias[J]. Gastroenterology, 2019, 157( 2): 365- 381. DOI: 10.1053/j.gastro.2019.04.050. |
| [2] |
Red Blood Cell Diseases(Anemia) Group of the Hematology Branch of the Chinese Medical Association. Expert consensus on the diagnosis and treatment of porphyrias in China(2020)[J]. Natl Med J China, 2020, 100( 14): 1051- 1056. DOI: 10.3760/cma.j.cn112137-20200219-00349. |
| [3] |
BUSTAD HJ, KALLIO JP, VORLAND M, et al. Acute intermittent Porphyria: An overview of therapy developments and future perspectives focusing on stabilisation of HMBS and proteostasis regulators[J]. Int J Mol Sci, 2021, 22( 2): 675. DOI: 10.3390/ijms22020675. |
| [4] |
ANDERSON KE. Acute hepatic porphyrias: Current diagnosis& management[J]. Mol Genet Metab, 2019, 128( 3): 219- 227. DOI: 10.1016/j.ymgme.2019.07.002. |
| [5] |
WANG B, BONKOVSKY HL, LIM JK, et al. AGA clinical practice update on diagnosis and management of acute hepatic porphyrias: Expert review[J]. Gastroenterology, 2023, 164( 3): 484- 491. DOI: 10.1053/j.gastro.2022.11.034. |
| [6] |
WANG B, RUDNICK S, CENGIA B, et al. Acute hepatic porphyrias: Review and recent progress[J]. Hepatol Commun, 2018, 3( 2): 193- 206. DOI: 10.1002/hep4.1297. |
| [7] |
YARRA P, FAUST D, BENNETT M, et al. Benefits of prophylactic heme therapy in severe acute intermittent porphyria[J]. Mol Genet Metab Rep, 2019, 19: 100450. DOI: 10.1016/j.ymgmr.2019.01.002. |
| [8] |
ZÜBARIOĞLU T, KıYKıM E, AKTUĞLU-ZEYBEK Ç. An overview of acute hepatic porphyrias: Clinical implications, diagnostic approaches, and management strategies[J]. Turk Arch Pediatr, 2023, 58( 1): 3- 9. DOI: 10.5152/TurkArchPediatr.2022.22301. |
| [9] |
PETRIDES PE. Therapy follows diagnosis: Old and new approaches for the treatment of acute porphyrias, what we know and what we should know[J]. Diagnostics, 2022, 12( 7): 1618. DOI: 10.3390/diagnostics12071618. |
| [10] |
TRABER GM, YU AM. RNAi-based therapeutics and novel RNA bioengineering technologies[J]. J Pharmacol Exp Ther, 2023, 384( 1): 133- 154. DOI: 10.1124/jpet.122.001234. |
| [11] |
YASUDA M, GAN L, CHEN B, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice[J]. Proc Natl Acad Sci U S A, 2014, 111( 21): 7777- 7782. DOI: 10.1073/pnas.1406228111. |
| [12] |
SARDH E, HARPER P, BALWANI M, et al. Phase 1 trial of an RNA interference therapy for acute intermittent Porphyria[J]. N Engl J Med, 2019, 380( 6): 549- 558. DOI: 10.1056/NEJMoa1807838. |
| [13] |
BALWANI M, SARDH E, VENTURA P, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent Porphyria[J]. N Engl J Med, 2020, 382( 24): 2289- 2301. DOI: 10.1056/NEJMoa1913147. |
| [14] |
LEE J, MELCH M, ROBBIE GJ. Pharmacokinetic-pharmacodynamic model of urinary δ-aminolevulinic acid reduction after givosiran treatment in patients with acute hepatic porphyria[J]. CPT Pharmacometrics Syst Pharmacol, 2023, 12( 6): 842- 852. DOI: 10.1002/psp4.12957. |
| [15] |
KUTER DJ, BONKOVSKY HL, MONROY S, et al. Efficacy and safety of givosiran for acute hepatic porphyria: Final results of the randomized phase III ENVISION trial[J]. J Hepatol, 2023, 79( 5): 1150- 1158. DOI: 10.1016/j.jhep.2023.06.013. |
| [16] |
MA CD, FAUST D, BONKOVSKY HL. Idiosyncratic drug-induced liver injury caused by givosiran in a patient with acute intermittent porphyria[J]. Mol Genet Metab Rep, 2022, 34: 100946. DOI: 10.1016/j.ymgmr.2022.100946. |
| [17] |
YASUDA M, KEEL S, BALWANI M. RNA interference therapy in acute hepatic porphyrias[J]. Blood, 2023, 142( 19): 1589- 1599. DOI: 10.1182/blood.2022018662. |
| [18] |
|
| [19] |
SARDH E, REJKJAER L, ANDERSSON DEH, et al. Safety, pharmacokinetics and pharmocodynamics of recombinant human porphobilinogen deaminase in healthy subjects and asymptomatic carriers of the acute intermittent porphyria gene who have increased porphyrin precursor excretion[J]. Clin Pharmacokinet, 2007, 46( 4): 335- 349. DOI: 10.2165/00003088-200746040-00006. |
| [20] |
FONTANELLAS A, ÁVILA MA, BERRAONDO P. Emerging therapies for acute intermittent porphyria[J]. Expert Rev Mol Med, 2016, 18: e17. DOI: 10.1017/erm.2016.18. |
| [21] |
CÓRDOBA KM, SERRANO-MENDIOROZ I, JERICÓ D, et al. Recombinant porphobilinogen deaminase targeted to the liver corrects enzymopenia in a mouse model of acute intermittent porphyria[J]. Sci Transl Med, 2022, 14( 627): eabc0700. DOI: 10.1126/scitranslmed.abc0700. |
| [22] |
JERICÓ D, CÓRDOBA KM, SAMPEDRO A, et al. Recent insights into the pathogenesis of acute Porphyria attacks and increasing hepatic PBGD as an etiological treatment[J]. Life, 2022, 12( 11): 1858. DOI: 10.3390/life12111858. |
| [23] |
|
| [24] |
PAÑEDA A, LOPEZ-FRANCO E, KAEPPEL C, et al. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman Primates: A potential therapy for acute intermittent porphyria[J]. Hum Gene Ther, 2013, 24( 12): 1007- 1017. DOI: 10.1089/hum.2013.166. |
| [25] |
D’AVOLA D, LÓPEZ-FRANCO E, SANGRO B, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria[J]. J Hepatol, 2016, 65( 4): 776- 783. DOI: 10.1016/j.jhep.2016.05.012. |
| [26] |
SERRANO-MENDIOROZ I, SAMPEDRO A, SERNA N, et al. Bioengineered PBGD variant improves the therapeutic index of gene therapy vectors for acute intermittent porphyria[J]. Hum Mol Genet, 2018, 27( 21): 3688- 3696. DOI: 10.1093/hmg/ddy283. |
| [27] |
SERRANO-MENDIOROZ I, SAMPEDRO A, ALEGRE M, et al. An inducible promoter responsive to different porphyrinogenic stimuli improves gene therapy vectors for acute intermittent Porphyria[J]. Hum Gene Ther, 2018, 29( 4): 480- 491. DOI: 10.1089/hum.2017.056. |
| [28] |
CÓRDOBA KM, JERICÓ D, SAMPEDRO A, et al. Messenger RNA as a personalized therapy: The moment of truth for rare metabolic diseases[J]. Int Rev Cell Mol Biol, 2022, 372: 55- 96. DOI: 10.1016/bs.ircmb.2022.03.005. |
| [29] |
JIANG L, BERRAONDO P, JERICÓ D, et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria[J]. Nat Med, 2018, 24( 12): 1899- 1909. DOI: 10.1038/s41591-018-0199-z. |
| [30] |
BUSTAD HJ, TOSKA K, SCHMITT C, et al. A pharmacological chaperone therapy for acute intermittent Porphyria[J]. Mol Ther, 2020, 28( 2): 677- 689. DOI: 10.1016/j.ymthe.2019.11.010. |
| [31] |
HALLOY F, IYER PS, GHIDINI A, et al. Repurposing of glycine transport inhibitors for the treatment of erythropoietic protoporphyria[J]. Cell Chem Biol, 2021, 28( 8): 1221- 1234. DOI: 10.1016/j.chembiol.2021.02.021. |
| [32] |
SOLARES I, TEJEDOR M, JERICÓ D, et al. Management of hyponatremia associated with acute porphyria-proposal for the use of tolvaptan[J]. Ann Transl Med, 2020, 8( 17): 1098. DOI: 10.21037/atm-20-1529. |
| [33] |
MROZEK S, ROUSSET D, GEERAERTS T. Pharmacotherapy of sodium disorders in neurocritical care[J]. Curr Opin Crit Care, 2019, 25( 2): 132- 137. DOI: 10.1097/MCC.0000000000000589. |
| [34] |
LI QY, REN Y, HOU JT, et al. Refractory hyponatremia caused by acute intermittent porphyria[J]. Chin J Endocrinol Metab, 2022, 38( 9): 815- 818. DOI: 10.3760/cma.j.cn311282-20211103-00699. |
| [35] |
PERI A. Management of hyponatremia: Causes, clinical aspects, differential diagnosis and treatment[J]. Expert Rev Endocrinol Metab, 2019, 14( 1): 13- 21. DOI: 10.1080/17446651.2019.1556095. |
| [36] |
SHANKAR J, BANFIELD J. Posterior reversible encephalopathy syndrome: A review[J]. Can Assoc Radiol J, 2017, 68( 2): 147- 153. DOI: 10.1016/j.carj.2016.08.005. |
| [37] |
ZHENG XP, LIU XJ, WANG Y, et al. Acute intermittent porphyria presenting with seizures and posterior reversible encephalopathy syndrome: Two case reports and a literature review[J]. Medicine, 2018, 97( 36): e11665. DOI: 10.1097/MD.0000000000011665. |
| [38] |
GOUYA L, VENTURA P, BALWANI M, et al. EXPLORE: A prospective, multinational, natural history study of patients with acute hepatic Porphyria with recurrent attacks[J]. Hepatology, 2020, 71( 5): 1546- 1558. DOI: 10.1002/hep.30936. |
| [39] |
HAVERKAMP T, BRONISCH O, KNÖSEL T, et al. Heterogeneous molecular behavior in liver tumors(HCC and CCA) of two patients with acute intermittent porphyria[J]. J Cancer Res Clin Oncol, 2023, 149( 6): 2647- 2655. DOI: 10.1007/s00432-022-04384-5. |
| [40] |
LISSING M, NOWAK G, ADAM R, et al. Liver transplantation for acute intermittent Porphyria[J]. Liver Transpl, 2021, 27( 4): 491- 501. DOI: 10.1002/lt.25959. |
| [41] |
STORJORD E, AIRILA-MÅNSSON S, KARLSEN K, et al. Dental and periodontal health in acute intermittent Porphyria[J]. Life, 2022, 12( 8): 1270. DOI: 10.3390/life12081270. |
| [42] |
MOORE AW 3rd, COKE JM. Acute porphyric disorders[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2000, 90( 3): 257- 262. DOI: 10.1067/moe.2000.107976. |
| [43] |
GUO YY, LUO M. Treatment of acute intermittent porphyria related to menstrual cycle with GnRH-A[J]. J Reprod Med, 2023, 32( 3): 438- 442. DOI: 10.3969/j.issn.1004-3845.2023.03.022. |
| [44] |
XU J, YI CH, HE JP, et al. Long-term remission of acute intermittent Porphyria treated with gonadotropin-releasing hormone analogues and estrogen: A case report[J]. Clin Lab, 2022, 68( 9). DOI: 10.7754/Clin.Lab.2022.211218. |
| [45] |
TSUDA M, OGAWA K, ENDOU T, et al. Absorption, metabolism, and excretion of[ 14C]dersimelagon, an investigational oral selective melanocortin 1 receptor agonist, in preclinical species and healthy volunteers[J]. Pharmacol Res Perspect, 2023, 11( 3): e01084. DOI: 10.1002/prp2.1084. |



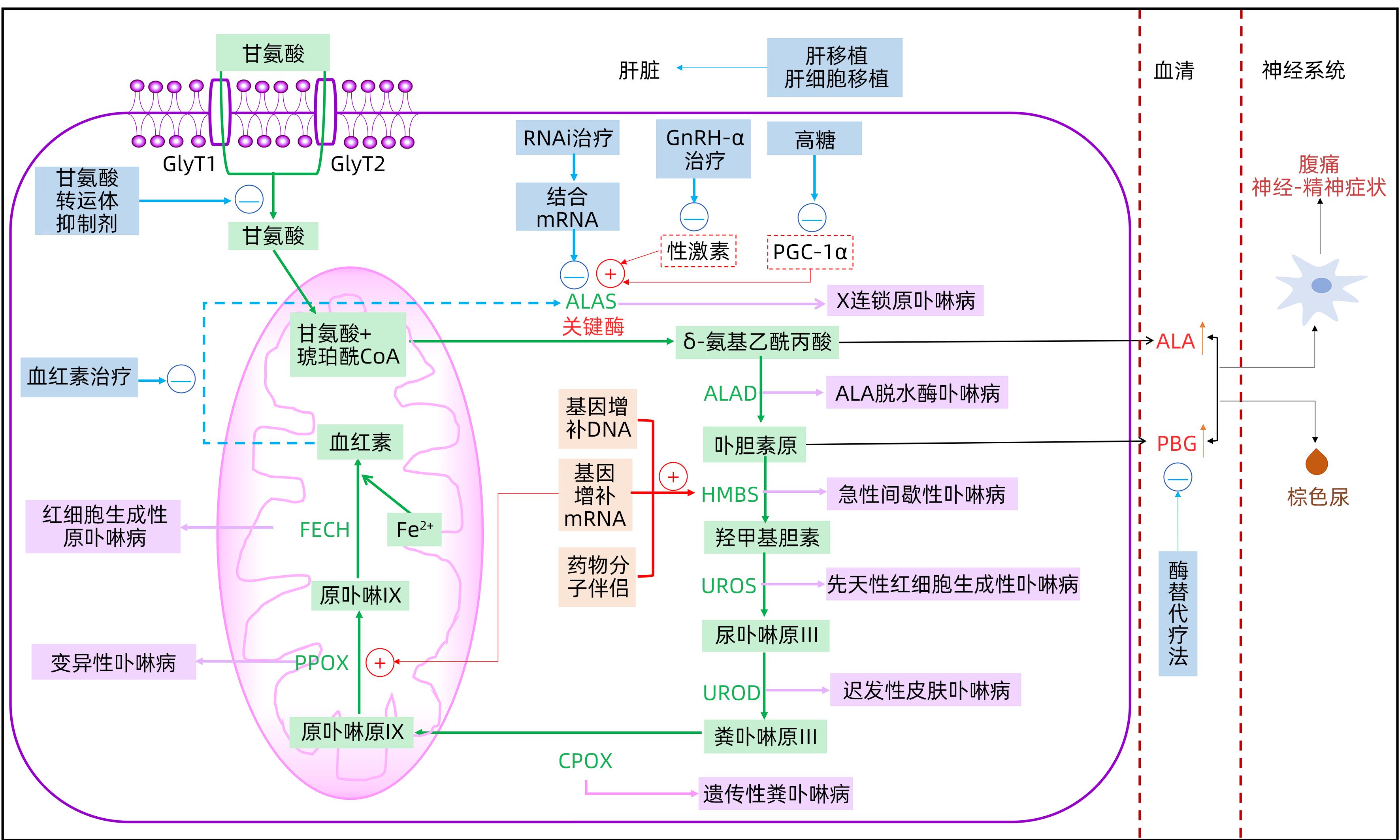




 DownLoad:
DownLoad: