阿司匹林在代谢相关脂肪性肝病中的作用
DOI: 10.12449/JCH260122
-
摘要: 代谢相关脂肪性肝病(MAFLD)是全球慢性肝病的主要类型,其发病率持续上升且呈年轻化趋势。目前,MAFLD的治疗方案主要依赖于生活方式干预及代谢合并症的管理,尚缺乏针对MAFLD本身的有效药物。阿司匹林作为一种经典的水杨酸类非甾体抗炎药,可通过调节脂质代谢、改善胰岛素抵抗、减轻肝脏炎症与氧化应激反应、抗肝纤维化及抑制肝细胞癌等多种作用机制,干预MAFLD的病理进程,具有预防疾病发生及延缓、逆转病情进展的价值。本文系统综述了阿司匹林在MAFLD治疗中的作用机制及安全性,以期为MAFLD患者提供更多的药物治疗选择。Abstract: Metabolic associated fatty liver disease (MAFLD) is the main type of chronic liver disease in the world, with an increasingly higher incidence rate and a younger age of onset. At present, the treatment of MAFLD mainly depends on lifestyle intervention and comorbidity management, and there is still a lack of effective drugs for MAFLD itself. As a classic nonsteroidal anti-inflammatory drug of the salicylic acid family, aspirin can intervene in the pathological process of MAFLD by regulating lipid metabolism, relieving insulin resistance, reducing liver inflammation and oxidative stress response, exerting an anti-liver fibrosis effect, and inhibiting hepatocellular carcinoma, and therefore, it has the value of preventing disease onset, delaying disease progression, and reversing disease condition. This article systematically reviews the mechanism of action and safety of aspirin in the treatment of MAFLD, in order to provide more drug treatment options for MAFLD patients.
-
Key words:
- Metabolic Associated Fatty Liver Disease /
- Aspirin /
- Drug Therapy
-
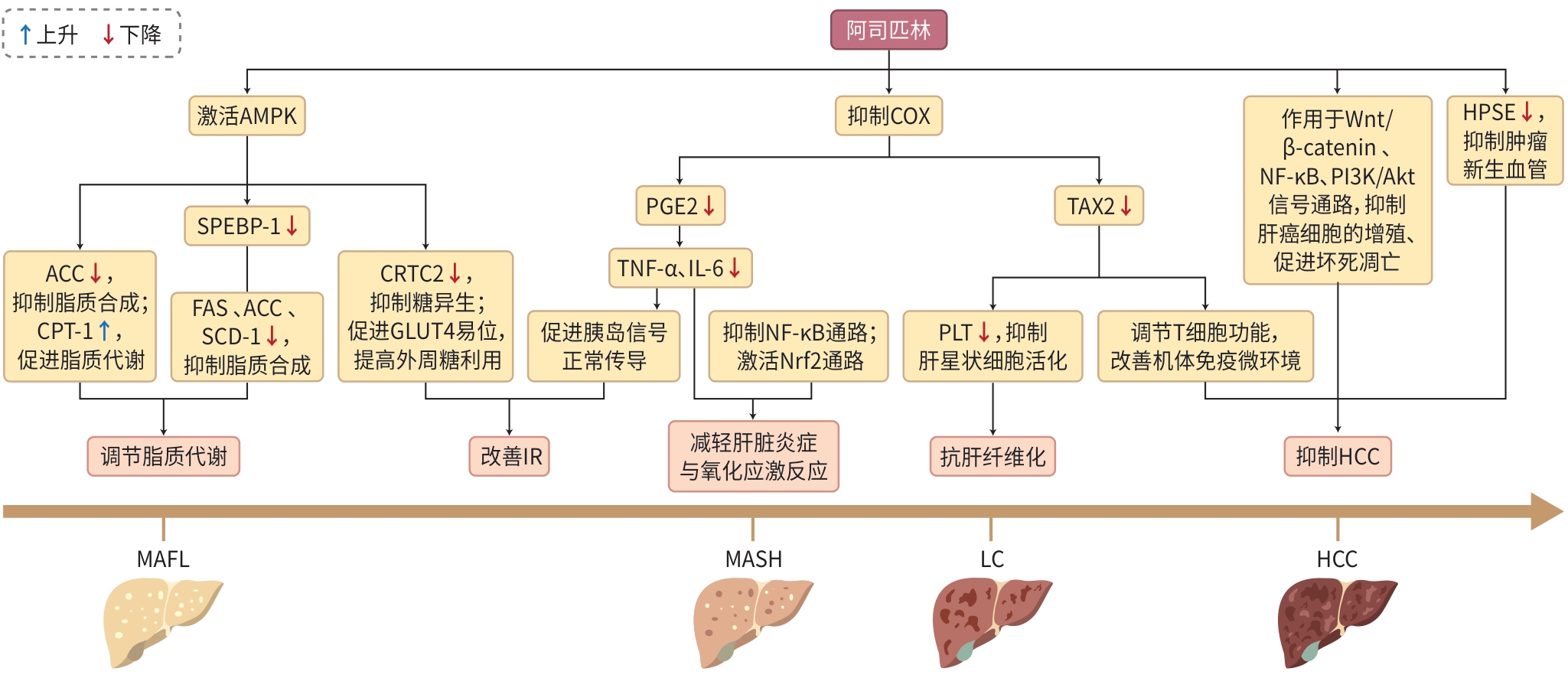
注: AMPK,AMP活化的蛋白质激酶;ACC,乙酰辅酶A羧化酶;CPT-1,肉碱棕榈酰转移酶-1;SPEBP-1,固醇调节元件结合蛋白-1;FAS,脂肪酸合成酶;SCD-1,硬脂酰辅酶A去饱和酶-1;CRTC2,CREB调节转录共激活因子2;GLUT4,葡萄糖转运蛋白4;COX,环氧合酶;PGE2,前列腺素E2;TNF-α,肿瘤坏死因子-α;IL-6,白细胞介素-6;NF-κB,核因子κB;Nrf2,核转录因子红系2相关因子2;TXA2,血栓素A2;PLT,血小板;Wnt/β-catenin,Wnt/β-连环蛋白;PI3K/Akt,磷脂酰肌醇3-激酶/蛋白激酶B;HPSE,乙酰肝素酶;MAFL,代谢相关脂肪肝;MASH,代谢相关脂肪性肝炎;LC,肝硬化;HCC,肝细胞癌。
图 1 阿司匹林在MAFLD中的作用
Figure 1. Role of aspirin in MAFLD
-
[1] European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease(MASLD)[J]. Obes Facts, 2024, 17( 4): 374- 444. DOI: 10.1159/000539371. [2] YOUNOSSI Z, ANSTEE QM, MARIETTI M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15( 1): 11- 20. DOI: 10.1038/nrgastro.2017.109. [3] POUWELS S, SAKRAN N, GRAHAM Y, et al. Non-alcoholic fatty liver disease(NAFLD): A review of pathophysiology, clinical management and effects of weight loss[J]. BMC Endocr Disord, 2022, 22( 1): 63. DOI: 10.1186/s12902-022-00980-1. [4] ELSHAER A, LIZAOLA-MAYO BC. Evaluating the role of aspirin in liver disease: Efficacy, safety, potential benefits and risks[J]. Life, 2024, 14( 12): 1701. DOI: 10.3390/life14121701. [5] SHEN H, SHAHZAD G, JAWAIRIA M, et al. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: A cross-sectional study from the Third National Health and Nutrition Examination Survey[J]. Aliment Pharmacol Ther, 2014, 40( 9): 1066- 1073. DOI: 10.1111/apt.12944. [6] SIMON TG, WILECHANSKY RM, STOYANOVA S, et al. Aspirin for metabolic dysfunction-associated steatotic liver disease without cirrhosis: A randomized clinical trial[J]. JAMA, 2024, 331( 11): 920- 929. DOI: 10.1001/jama.2024.1215. [7] KARIN M, KIM JY. MASH as an emerging cause of hepatocellular carcinoma: Current knowledge and future perspectives[J]. Mol Oncol, 2025, 19( 2): 275- 294. DOI: 10.1002/1878-0261.13685. [8] RONG L, ZOU JY, RAN W, et al. Advancements in the treatment of non-alcoholic fatty liver disease(NAFLD)[J]. Front Endocrinol, 2022, 13: 1087260. DOI: 10.3389/fendo.2022.1087260. [9] HARRISON SA, BEDOSSA P, GUY CD, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis[J]. N Engl J Med, 2024, 390( 6): 497- 509. DOI: 10.1056/NEJMoa2309000. [10] HE ZX, PENG Y, DUAN WT, et al. Aspirin regulates hepatocellular lipid metabolism by activating AMPK signaling pathway[J]. J Toxicol Sci, 2015, 40( 1): 127- 136. DOI: 10.2131/jts.40.127. [11] STEINBERG GR, CARLING D. AMP-activated protein kinase: The current landscape for drug development[J]. Nat Rev Drug Discov, 2019, 18( 7): 527- 551. DOI: 10.1038/s41573-019-0019-2. [12] LI Y, XU SQ, MIHAYLOVA MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice[J]. Cell Metab, 2011, 13( 4): 376- 388. DOI: 10.1016/j.cmet.2011.03.009. [13] SHIMANO H, SATO R. SREBP-regulated lipid metabolism: Convergent physiology: Divergent pathophysiology[J]. Nat Rev Endocrinol, 2017, 13( 12): 710- 730. DOI: 10.1038/nrendo.2017.91. [14] LI AQ, ZHAO PR, ZHAO YQ, et al. Mechanism of action of sterol regulatory element-binding proteins in nonalcoholic fatty liver disease and related therapeutic targets[J]. J Clin Hepatol, 2024, 40( 7): 1459- 1465. DOI: 10.12449/JCH240726.李安琪, 赵佩然, 赵玉强, 等. 固醇调节元件结合蛋白(SREBP)在非酒精性脂肪性肝病中的作用机制及治疗靶点[J]. 临床肝胆病杂志, 2024, 40( 7): 1459- 1465. DOI: 10.12449/JCH240726. [15] ROY S, BHOWMIK DR, BEGUM R, et al. Aspirin attenuates the expression of adhesion molecules, risk of obesity, and adipose tissue inflammation in high-fat diet-induced obese mice[J]. Prostaglandins Other Lipid Mediat, 2022, 162: 106664. DOI: 10.1016/j.prostagl-andins.2022.106664. [16] KAMANI L, SIDDIQUI M, RAHAT A. Frequency of insulin resistance among non-diabetic patients with non-alcoholic fatty liver disease using HOMA-IR: An experience of a tertiary care hospital in Karachi, Pakistan[J]. BMC Gastroenterol, 2025, 25( 1): 259. DOI: 10.1186/s12876-025-03790-6. [17] YAO ZY, GONG Y, CHEN WB, et al. Upregulation of WDR6 drives hepatic de novo lipogenesis in insulin resistance in mice[J]. Nat Metab, 2023, 5( 10): 1706- 1725. DOI: 10.1038/s42255-023-00896-7. [18] LIU FM, WANG Q, QIAN YZ, et al. Research progress of Adenosine 5′-monophosphate-activated protein kinase in the regulation of glycolipid metabolism[J]. Chin J Biotechnol, 2019, 35( 6): 1021- 1028. DOI: 10.13345/j.cjb.180529.刘凡铭, 王琪, 钱昱臻, 等. 腺苷酸活化蛋白激酶在糖脂代谢调控中的研究进展[J]. 生物工程学报, 2019, 35( 6): 1021- 1028. DOI: 10.13345/j.cjb.180529. [19] RUMORE MM, KIM KS. Potential role of salicylates in type 2 diabetes[J]. Ann Pharmacother, 2010, 44( 7-8): 1207- 1221. DOI: 10.1345/aph.1M483. [20] REHMAN K, AKASH MSH, LIAQAT A, et al. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus[J]. Crit Rev Eukaryot Gene Expr, 2017, 27( 3): 229- 236. DOI: 10.1615/CritRevEukaryotGeneExpr.2017019712. [21] KAJANI S, CURLEY S, O’REILLY ME, et al. Sodium salicylate rewires hepatic metabolic pathways in obesity and attenuates IL-1β secretion from adipose tissue: The implications for obesity-impaired reverse cholesterol transport[J]. Mol Metab, 2022, 56: 101425. DOI: 10.1016/j.molmet.2021.101425. [22] ALEGBELEYE BJ, AKPOVESO OP, MOHAMMED RK, et al. Pharmacology, pharmaceutics and clinical use of aspirin: A narrative review[J]. J Drug Delivery Ther, 2020, 10( 5-s): 236- 253. DOI: 10.22270/jDDT.v10i5-s.4351. [23] LIU T, ZHANG LY, JOO D, et al. NF-κB signaling in inflammation[J]. Signal Transduct Target Ther, 2017, 2: 17023. DOI: 10.1038/sigtrans.2017.23. [24] BALTAZAR MT, DINIS-OLIVEIRA RJ, DUARTE JA, et al. Antioxidant properties and associated mechanisms of salicylates[J]. Curr Med Chem, 2011, 18( 21): 3252- 3264. DOI: 10.2174/092986711796391552. [25] SIMON TG, HENSON J, OSGANIAN S, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2019, 17( 13): 2776- 2784. DOI: 10.1016/j.cgh.2019.04.061. [26] LI L, YU JX, ZHOU ZW. Association between platelet indices and non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Rev Esp Enferm Dig, 2024, 116( 5): 264- 273. DOI: 10.17235/reed.2022.9142/2022. [27] JIANG QR, MAO RC, WU JW, et al. Platelet activation during chronic hepatitis B infection exacerbates liver inflammation and promotes fibrosis[J]. J Med Virol, 2020, 92( 12): 3319- 3326. DOI: 10.1002/jmv.25641. [28] TAN DJH, NG CH, LIN SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis[J]. Lancet Oncol, 2022, 23( 4): 521- 530. DOI: 10.1016/S1470-2045(22)00078-X. [29] LANGE NF, RADU P, DUFOUR JF. Prevention of NAFLD-associated HCC: Role of lifestyle and chemoprevention[J]. J Hepatol, 2021, 75( 5): 1217- 1227. DOI: 10.1016/j.jhep.2021.07.025. [30] RICCIOTTI E, WANGENSTEEN KJ, FITZGERALD GA. Aspirin in hepatocellular carcinoma[J]. Cancer Res, 2021, 81( 14): 3751- 3761. DOI: 10.1158/0008-5472.CAN-21-0758. [31] WANG YF, FENG JY, ZHAO LN, et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-κB p65-activated SLC7A11 transcription[J]. Acta Pharmacol Sin, 2023, 44( 8): 1712- 1724. DOI: 10.1038/s41401-023-01062-1. [32] DAI XY, YAN J, FU XH, et al. Aspirin inhibits cancer metastasis and angiogenesis via targeting heparanase[J]. Clin Cancer Res, 2017, 23( 20): 6267- 6278. DOI: 10.1158/1078-0432.CCR-17-0242. [33] YANG J, YAMASHITA-KANEMARU Y, MORRIS BI, et al. Aspirin prevents metastasis by limiting platelet TXA(2) suppression of T cell immunity[J]. Nature, 2025, 640( 8060): 1052- 1061. DOI: 10.1038/s41586-025-08626-7. [34] YAN LJ, YAO SY, LI HC, et al. Efficacy and safety of aspirin for prevention of hepatocellular carcinoma: An updated meta-analysis[J]. J Clin Transl Hepatol, 2022, 10( 5): 835- 846. DOI: 10.14218/JCTH.2021.00257. [35] LEE TY, HSU YC, HO HJ, et al. Daily aspirin associated with a reduced risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: A population-based cohort study[J]. EClinicalMedicine, 2023, 61: 102065. DOI: 10.1016/j.eclinm.2023.102065. [36] SOSTRES C, LANAS A. Gastrointestinal effects of aspirin[J]. Nat Rev Gastroenterol Hepatol, 2011, 8( 7): 385- 394. DOI: 10.1038/nrgastro.2011.97. -



 PDF下载 ( 668 KB)
PDF下载 ( 668 KB)

 下载:
下载:


