DEAD-box解旋酶3X-连锁(DDX3X)在免疫介导性肝损伤小鼠模型和HBV相关肝损伤患者中的表达分析
DOI: 10.12449/JCH260116
Role of endoplasmic reticulum stress-mediated DEAD-box helicase 3 X-linked in a mouse model of concanavalin A-induced immune-mediated liver injury
-
摘要:
目的 探讨DEAD-box解旋酶3X-连锁(DDX3X)在免疫介导性肝损伤(ILI)中的作用,并阐明其通过调控内质网应激(ERS)依赖性凋亡通路的作用机制及其与乙型肝炎临床进展的相关性。 方法 采用刀豆蛋白A(ConA)尾静脉注射建立小鼠ILI模型;对肝细胞特异性DDX3X敲除小鼠(DDX3XΔHep)和DDX3X-flox小鼠(DDX3Xfl/fl)分别尾静脉注射PBS(对照组)和不同浓度的ConA,通过Log-rank生存分析、血清天冬氨酸氨基转移酶(AST)及丙氨酸氨基转移酶(ALT)检测、肝组织苏木精-伊红(HE)染色评估肝损伤严重程度;采用实时荧光定量PCR(qRT-PCR)和蛋白质印迹法(Western Blot)检测肝组织中葡萄糖调节蛋白78(GRP78)、CCAAT/增强子结合蛋白同源蛋白(CHOP)及DDX3X的表达;通过腹腔注射4-苯基丁酸(4-PBA,100 mg/kg)抑制ERS;收集北京佑安医院2019—2023年健康对照、慢性乙型肝炎及HBV相关肝衰竭(HBV-LF)患者血清样本各30例,肝组织样本各6例,通过酶联免疫吸附分析检测血清DDX3X水平,qRT-PCR/Western Blot分析肝组织靶标表达。计量资料多组间比较采用单因素方差分析,进一步两两比较使用LSD-t检验。采用Kaplan-Meier法绘制生存曲线。 结果 相较于正常对照组,ConA诱导肝损伤后小鼠肝脏DDX3X表达显著升高(P<0.05)。在致死剂量ConA处理后,DDX3XΔHep小鼠72 h生存率为55%,显著高于DDX3Xfl/fl对照组的20%(P<0.05),血清ALT和AST的水平亦同步显著降低(P值均<0.000 1),ERS标志物GRP78与CHOP表达同步下调(P<0.05)。在ConA诱导的小鼠肝损伤中,与PBS预处理组相比,4-PBA预处理抑制ERS不仅减轻了ConA诱导的肝损伤(ALT和AST降低,P值均<0.001),亦使肝脏DDX3X的mRNA与蛋白表达降低(P值均<0.01)。临床样本分析结果中,慢性乙型肝炎及HBV-LF患者肝组织DDX3X的mRNA和蛋白表达均高于健康对照(P值均<0.01),HBV-LF患者血清DDX3X水平显著升高(P<0.000 1)。 结论 DDX3X通过调控ERS依赖性凋亡通路(GRP78/CHOP)加重ILI,其表达与乙型肝炎疾病进展相关,可作为潜在治疗靶点。 Abstract:Objective To investigate the role of DEAD-box helicase 3 X-linked (DDX3X) in immune-mediated liver injury (ILI), and to clarify its mechanism by regulating endoplasmic reticulum stress (ERS)-dependent apoptotic pathway and its association with the clinical progression of hepatitis B. Methods Mice were given injection of concanavalin A (ConA) via the caudal vein to establish a model of ILI, PBS (control group) and different concentrations of ConA were injected into the tail vein of hepatocyte-specific DDX3X-knockout mice (DDX3XΔHep and DDX3X-flox mice (DDX3Xfl/fl), respectively.. The log-rank survival analysis, measurement of the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and HE staining of liver tissue were performed to assess liver injury, and qRT-PCR and Western Blot were used to measure the mRNA and protein expression levels of glucose-regulated protein 78 (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), and DDX3X in liver tissue. Intraperitoneal injection of 4-phenylbutyric acid (4-PBA, 100 mg/kg) was performed to inhibit ERS. Serum samples (n=30) and liver tissue samples (n=6) were collected from healthy controls, chronic hepatitis B (CHB) patients, and hepatitis B virus-associated liver failure (HBV-LF) patients; ELISA was used to measure the serum level of DDX3X, and qRT-PCR/Western Blot was used to analyze the expression of targets in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the control group of mice, the expression of DDX3X in the liver of mice induced by ConA was significantly increased after liver injury (P<0.05), and hepatocyte-specific DDX3X knockout increased the 72-hour survival rate of mice by 55% (compared with 20% in the DDX3Xfl/fl group), with significant reductions in the serum levels of ALT and AST (P<0.000 1) and the expression levels of the ERS markers GRP78 and CHOP (P<0.05). After ERS was inhibited by 4-PBA, there was alleviation of liver injury (with reductions in ALT and AST, P <0.001) and a reduction in DDX3X expression (P<0.01). The analysis of clinical samples showed that the mRNA and protein expression levels of liver DDX3X in CHB patients and HBV-LF patients were significantly higher than those in healthy controls (all P<0.01), and there was a significant increase in the serum level of DDX3X in HBV-LF patients (P<0.000 1). Conclusion DDX3X exacerbates ILI by regulating the ERS-dependent apoptotic pathway (GRP78/CHOP), and its expression is associated with the progression of hepatitis B. Therefore, it can be used as a potential therapeutic target. -
注: ConA,刀豆蛋白A;DDX3X,DEAD-box解旋酶3X-连锁;ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;ERS,内质网应激;PBS,磷酸盐缓冲溶液;4-PBA,4-苯基丁酸;CHOP,CCAAT增强子结合蛋白同源蛋白;GRP78,葡萄糖调节蛋白78;CHB,慢性乙型肝炎;HBV-LF,乙型肝炎病毒相关肝衰竭。
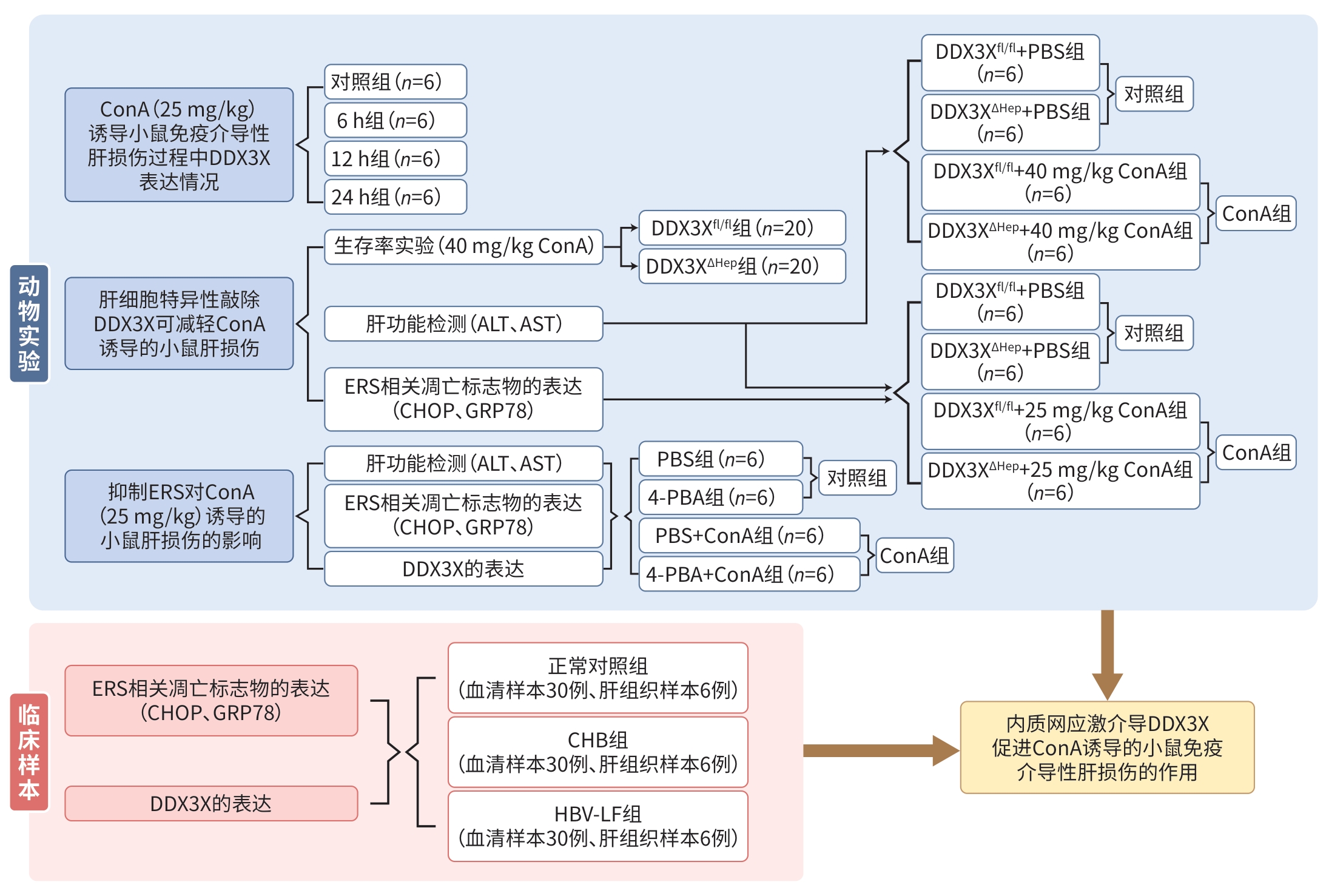
图 1 ERS介导DDX3X促进ConA诱导的小鼠ILI的作用研究流程图
Figure 1. Study flowchart of the role of ERS in mediating DDX3X-promoted immune-mediated liver injury induced by ConA in mice
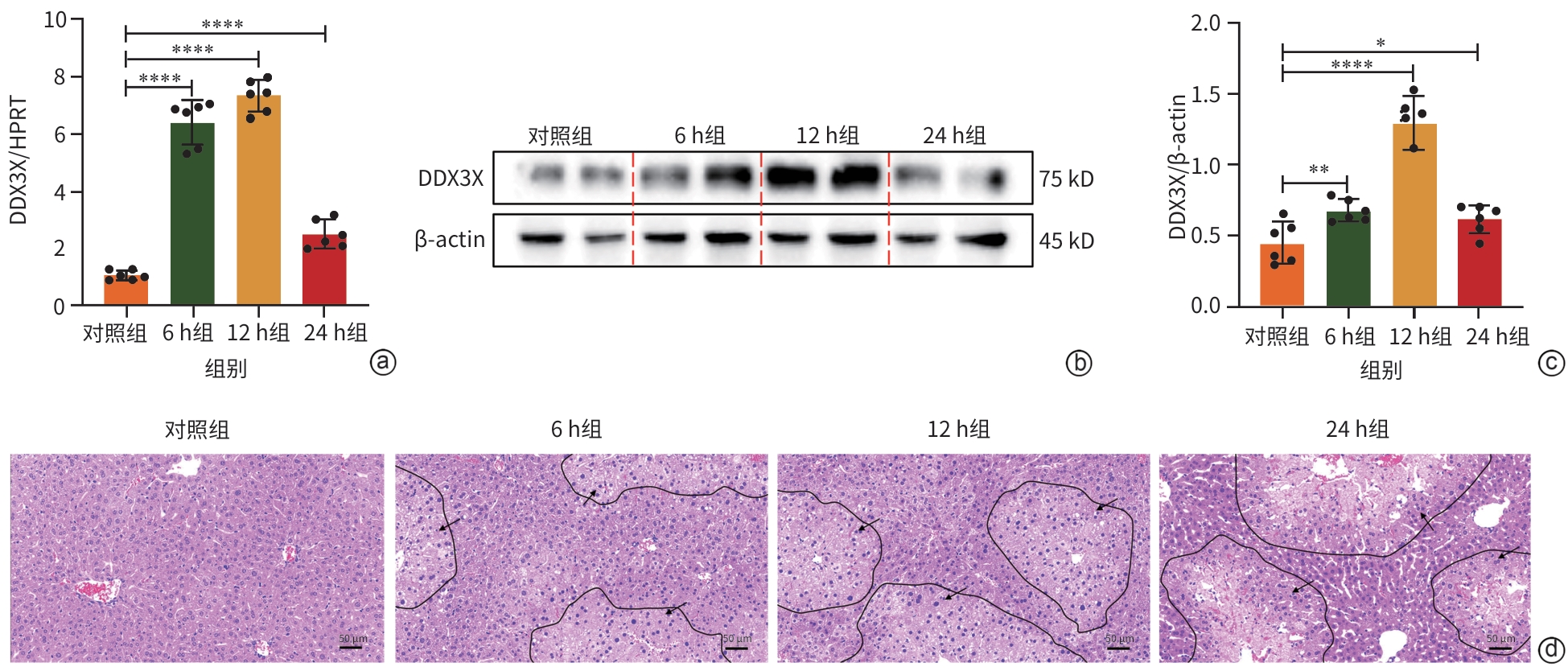
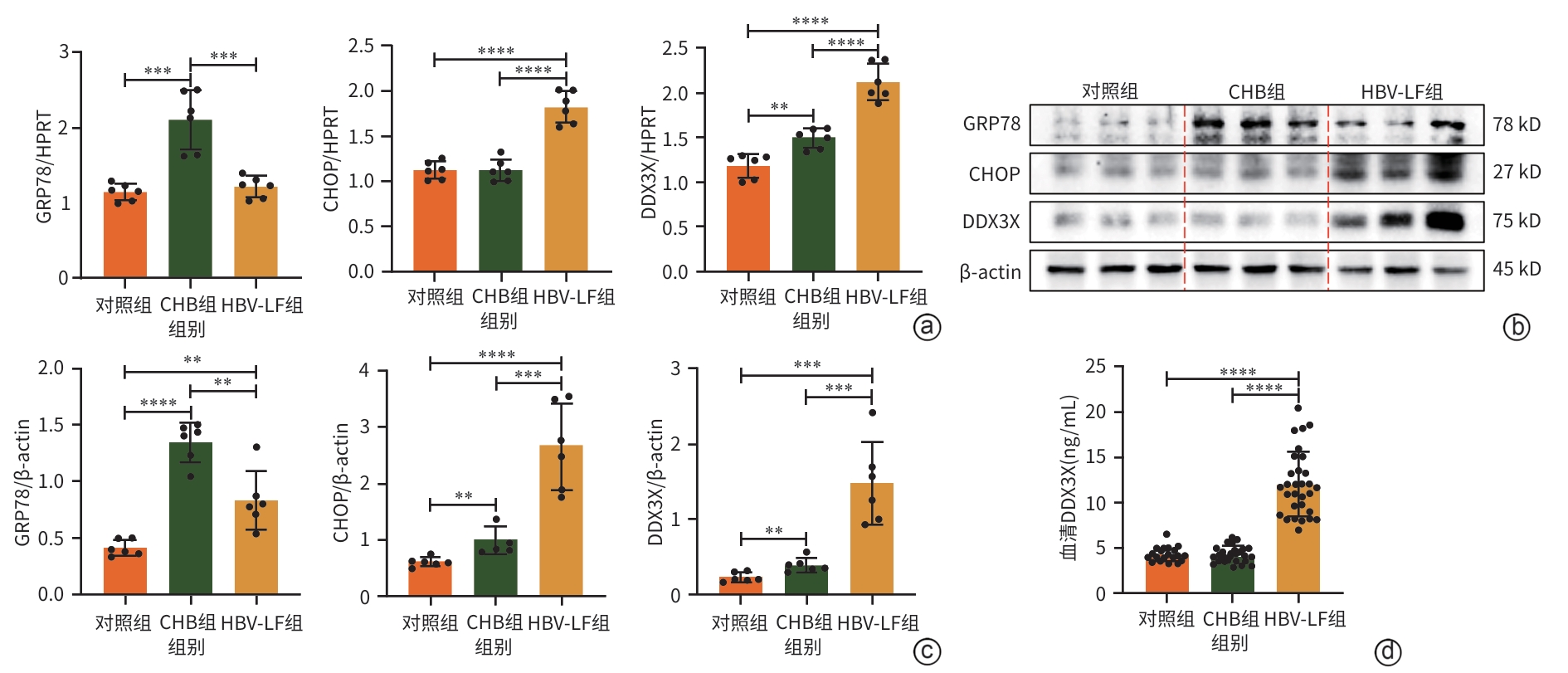
注: a,qRT-PCR检测DDX3X的mRNA水平;b,通过ImageJ测定Western Blot图像灰度值,并进行定量;c,Western Blot检测DDX3X的蛋白表达水平;d,肝组织病理学结果(HE染色,×10),黑色箭头处为损伤区域。*P<0.05,**P<0.01,****P<0.000 1。DDX3X,DEAD-box解旋酶3X-连锁;HPRT,次黄嘌呤磷酸核糖基转移酶;ConA,刀豆蛋白A;β-actin,β-肌动蛋白。
图 2 DDX3X在ConA诱导的肝损伤期间的表达情况
Figure 2. Expression of DDX3X in the liver during liver injury induced by ConA
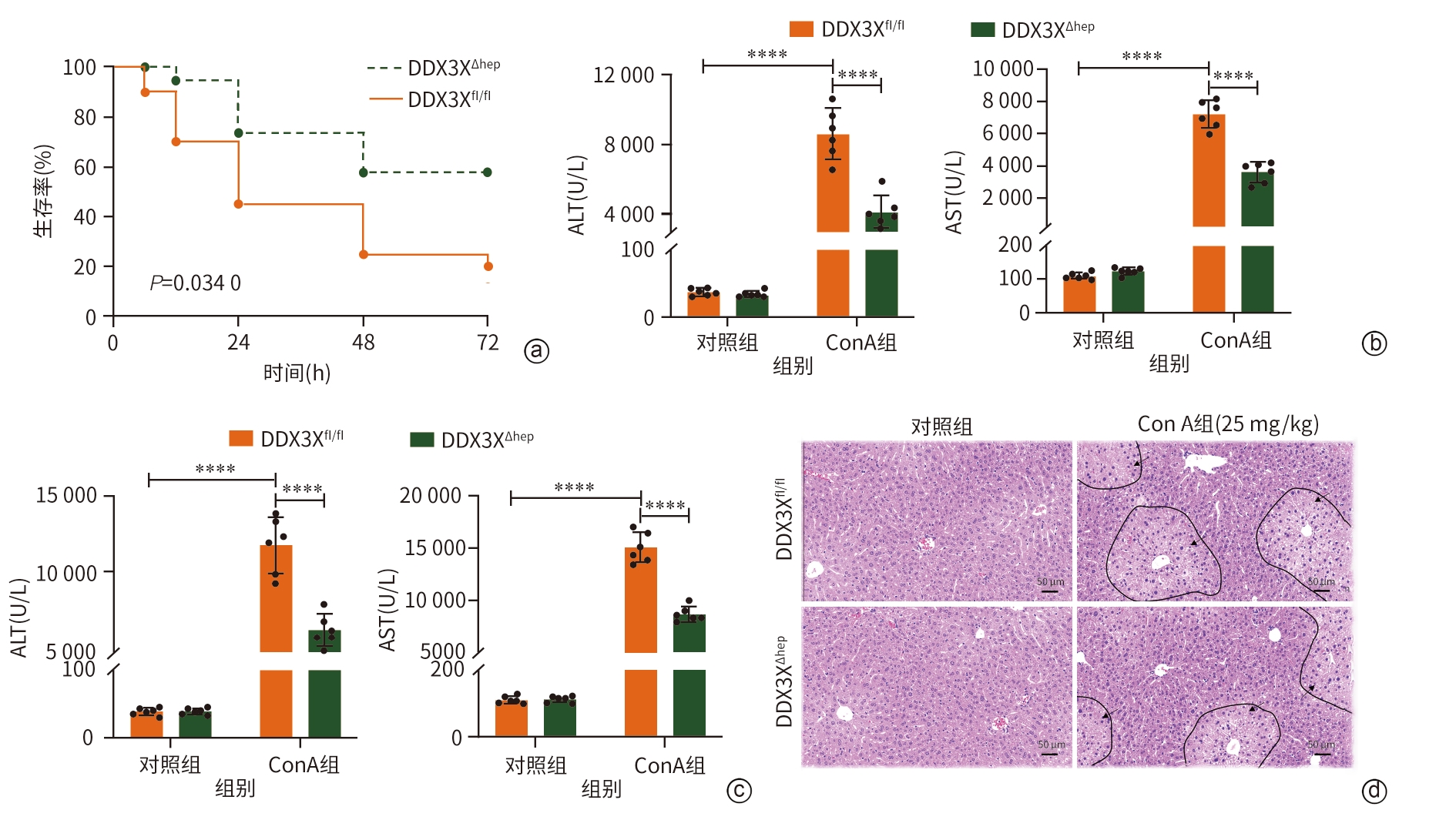
注: a,DDX3Xfl/fl和DDX3XΔHep小鼠用ConA(40 mg/kg)处理72 h生存曲线;b,DDX3Xfl/fl和DDX3XΔHep小鼠用ConA(25 mg/kg)处理8 h后ALT、AST水平;c,DDX3Xfl/fl和DDX3XΔHep小鼠用ConA(40 mg/kg)处理8 h后ALT、AST水平;d,肝组织病理学结果(HE染色,×10),黑色箭头处为损伤区域。****P<0.000 1。ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;DDX3X,DEAD-box解旋酶3X-连锁;DDX3Xfl/fl,DDX3X基因的野生型等位基因型小鼠;DDX3XΔHep,肝细胞特异性DDX3X敲除小鼠;ConA,刀豆蛋白A。
图 3 肝细胞特异性敲除DDX3X可改善ConA诱导的小鼠肝损伤
Figure 3. Hepatocellular specific knockout of DDX3X can improve liver injury induced by ConA in mice
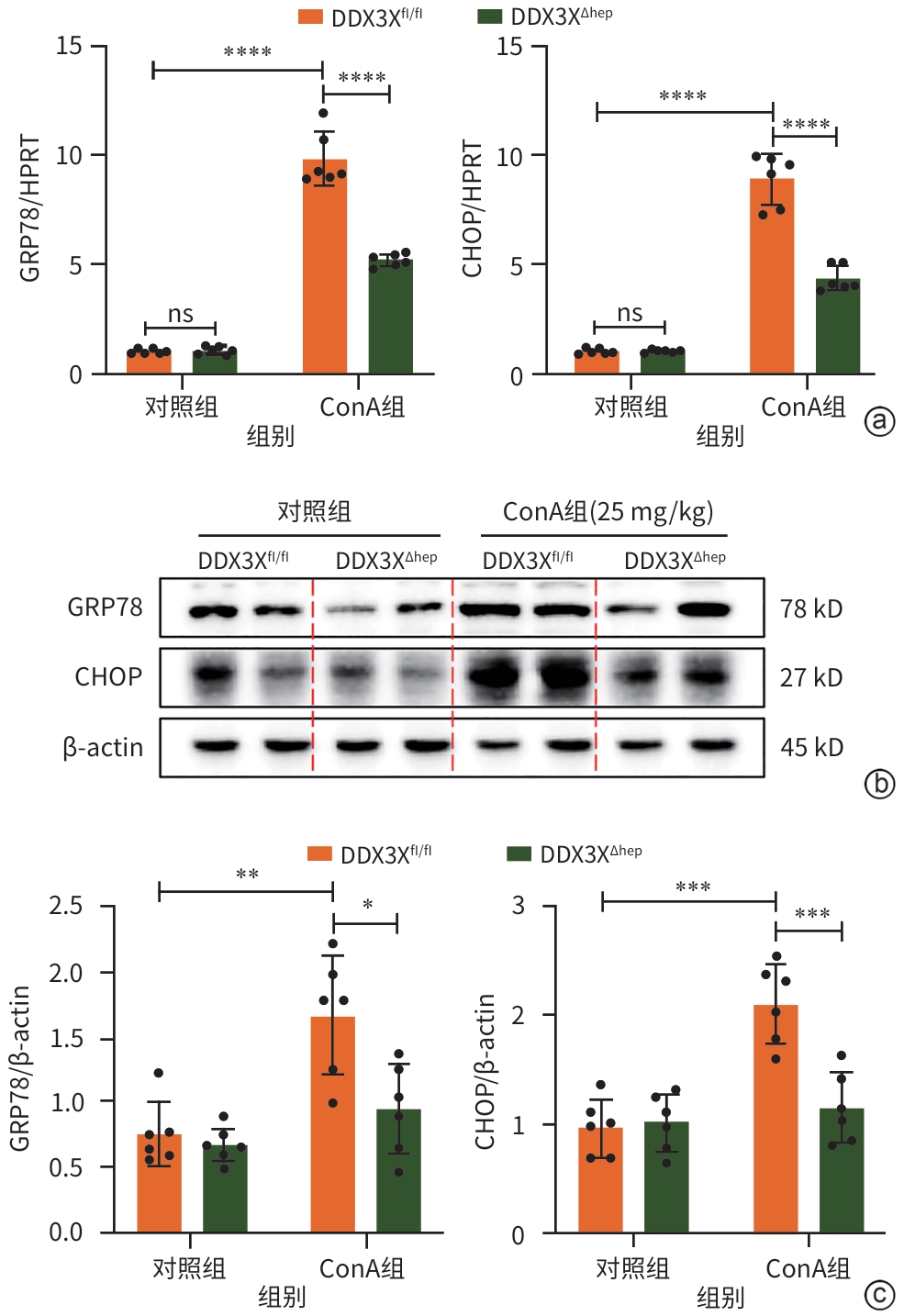
注: a,GRP78和CHOP的mRNA水平;b,Western Blot检测结果;c,GRP78和CHOP的蛋白表达水平。*P<0.05,**P<0.01,***P<0.001,****P<0.000 1。GRP78,葡萄糖调节蛋白78;HPRT,次黄嘌呤磷酸核糖基转移酶;DDX3X,DEAD-box解旋酶3X-连锁;CHOP,CCAAT增强子结合蛋白同源蛋白;β-actin,β-肌动蛋白;ConA,刀豆蛋白;DDX3Xfl/fl,DDX3X基因的野生型等位基因型小鼠;DDX3XΔHep,肝细胞特异性DDX3X敲除小鼠。
图 4 肝细胞特异性敲除DDX3X减少ERS相关凋亡标志物的表达
Figure 4. Hepatocellular specific knockout of DDX3X reduces the expression of ERS-related apoptotic markers
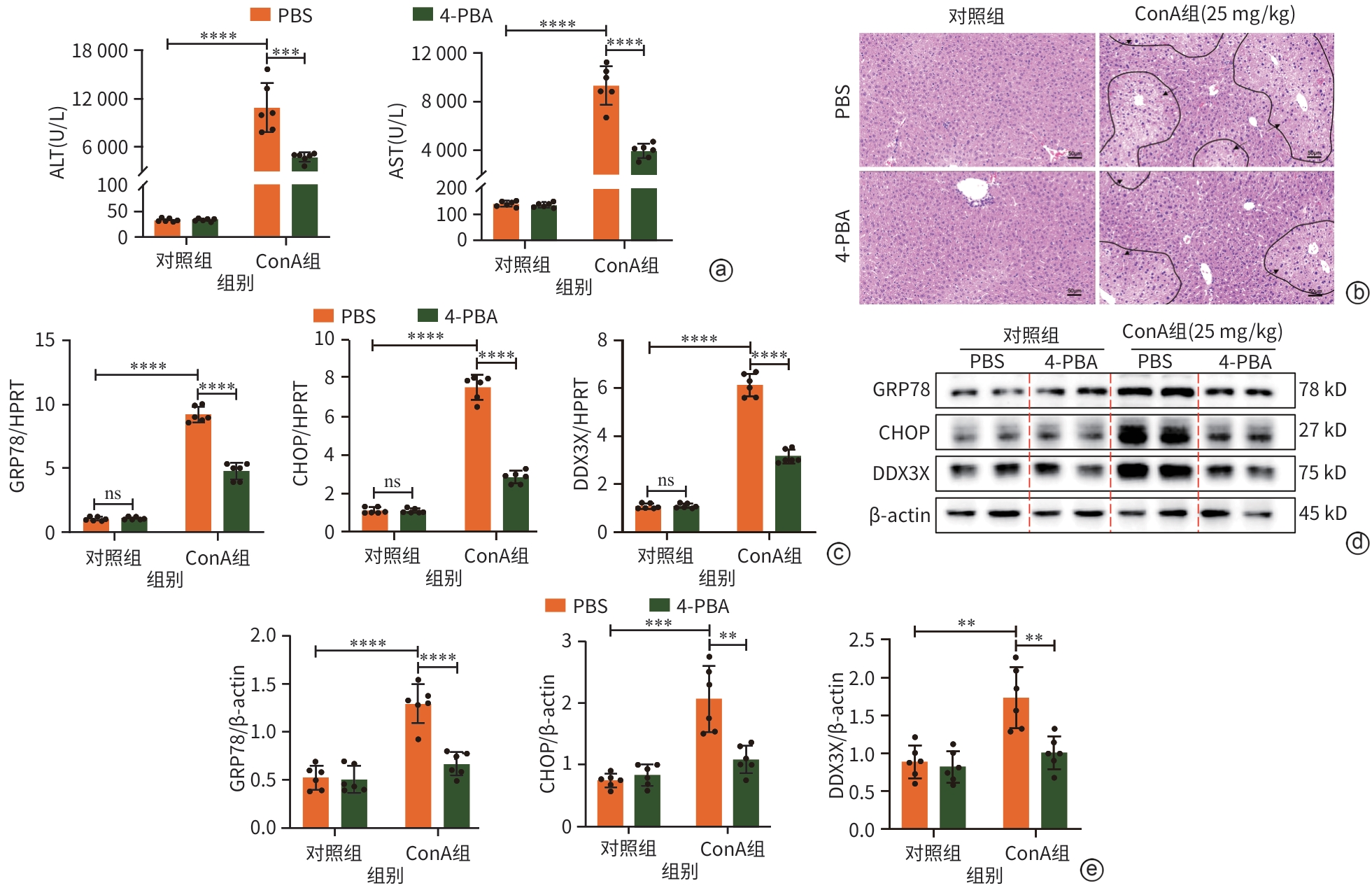
注: a,血清ALT和AST水平;b,肝组织病理学结果(HE染色,×10),黑色箭头处为损伤区域;c,GRP78、CHOP、DDX3X的mRNA水平;d,Western Blot检测结果;e,GRP78、CHOP、DDX3X的蛋白表达水平。**P<0.01,***P<0.001,****P<0.000 1。ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;4-PBA,4-苯基丁酸;PBS,磷酸盐缓冲溶液;ConA,刀豆蛋白A;GRP78,葡萄糖调节蛋白78;HPRT,次黄嘌呤磷酸核糖基转移酶;CHOP,CCAAT增强子结合蛋白同源蛋白;DDX3X,DEAD-box解旋酶3X-连锁;β-actin,β-肌动蛋白。
图 5 抑制ERS可下调ConA诱导的小鼠肝损伤中DDX3X的表达
Figure 5. Inhibition of ERS significantly reduced the expression of DDX3X in ConA-induced liver injury in mice
表 1 引物序列
Table 1. Primer sequence
基因 正向(5′-3′) 反向(5′-3′) HPRT(小鼠) AGTCCCAGCGTCGTGATTAG GCCTCCCATCTCCTTCATGA DDX3X(小鼠) GGCCGTGGAGATAGAAGTGG TGCACTGCCAATTCTCTCGT GRP78(小鼠) CTTTGATCAGCGGGTCATGG AGCTCTTCAAATTTGGCCCG CHOP(小鼠) TCACTACTCTTGACCCTGCG ACTGACCACTCTGTTTCCGT HPRT(人) TTCCTCCTCCTGAGCAGTCA ATCCAACACTTCGTGGGGTC DDX3X(人) GGGTGTCAAACTTCAACCGC TCCAGTCACCGGCATAGAGA GRP78(人) TAGCGTATGGTGCTGCTGTC TTTGTCAGGGGTCTTTCACC CHOP(人) TGGAAGCCTGGTATGAGGAC TGTGACCTCTGCTGGTTCTG 注:HPRT,次黄嘌呤磷酸核糖基转移酶;DDX3X,DEAD-box解旋酶3X-连锁;GRP78,葡萄糖调节蛋白78;CHOP,CCAAT增强子结合蛋白同源蛋白。
-
[1] ROBINSON MW, HARMON C, O’FARRELLY C. Liver immunology and its role in inflammation and homeostasis[J]. Cell Mol Immunol, 2016, 13( 3): 267- 276. DOI: 10.1038/cmi.2016.3. [2] HOOFNAGLE JH, BJÖRNSSON ES. Drug-induced liver injury-types and phenotypes[J]. N Engl J Med, 2019, 381( 3): 264- 273. DOI: 10.1056/NEJMra1816149. [3] LONGHI MS, MA Y, MIELI-VERGANI G, et al. Aetiopathogenesis of autoimmune hepatitis[J]. J Autoimmun, 2010, 34( 1): 7- 14. DOI: 10.1016/j.jaut.2009.08.010. [4] ADAMS DH, JU C, RAMAIAH SK, et al. Mechanisms of immune-mediated liver injury[J]. Toxicol Sci, 2010, 115( 2): 307- 321. DOI: 10.1093/toxsci/kfq009. [5] NABEKURA T, MATSUO S, SHIBUYA A. Concanavalin-A-induced acute liver injury in mice[J]. Curr Protoc, 2024, 4( 8): e1117. DOI: 10.1002/cpz1.1117. [6] TIEGS G, HENTSCHEL J, WENDEL A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A[J]. J Clin Invest, 1992, 90( 1): 196- 203. DOI: 10.1172/JCI115836. [7] CUI Y, QIAO FJ, QIU JH, et al. Effect of ganoderic acid A on a mouse model of concanavalin A-induced acute immune liver injury and its mechanism[J]. J Clin Hepatol, 2024, 40( 12): 2415- 2423. DOI: 10.12449/JCH241211.崔怡, 乔凤杰, 邱嘉昊, 等. 灵芝酸A对刀豆球蛋白A诱导的急性免疫性肝损伤小鼠模型的影响及其作用机制[J]. 临床肝胆病杂志, 2024, 40( 12): 2415- 2423. DOI: 10.12449/JCH241211. [8] HETZ C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond[J]. Nat Rev Mol Cell Biol, 2012, 13( 2): 89- 102. DOI: 10.1038/nrm3270. [9] OAKES SA, PAPA FR. The role of endoplasmic reticulum stress in human pathology[J]. Annu Rev Pathol, 2015, 10: 173- 194. DOI: 10.1146/annurev-pathol-012513-104649. [10] LEBEAUPIN C, VALLÉE D, HAZARI Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease[J]. J Hepatol, 2018, 69( 4): 927- 947. DOI: 10.1016/j.jhep.2018.06.008. [11] YAN CY, HU WT, TU JQ, et al. Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease[J]. J Transl Med, 2023, 21( 1): 300. DOI: 10.1186/s12967-023-04166-8. [12] HU TY, WANG JY, LI WX, et al. Endoplasmic reticulum stress in hepatitis B virus and hepatitis C virus infection[J]. Viruses, 2022, 14( 12): 2630. DOI: 10.3390/v14122630. [13] PU SS, PAN YY, ZHANG Q, et al. Endoplasmic reticulum stress and mitochondrial stress in drug-induced liver injury[J]. Molecules, 2023, 28( 7): 3160. DOI: 10.3390/molecules28073160. [14] MALHI H, KAUFMAN RJ. Endoplasmic reticulum stress in liver disease[J]. J Hepatol, 2011, 54( 4): 795- 809. DOI: 10.1016/j.jhep.2010.11.005. [15] JIANG JL, ZHOU YY, ZHONG WW, et al. Uridine diphosphate glucuronosyltransferase 1A1 prevents the progression of liver injury[J]. World J Gastroenterol, 2024, 30( 9): 1189- 1212. DOI: 10.3748/wjg.v30.i9.1189. [16] de BISSCHOP G, AMEUR M, ULRYCK N, et al. HIV-1 gRNA, a biological substrate, uncovers the potency of DDX3X biochemical activity[J]. Biochimie, 2019, 164: 83- 94. DOI: 10.1016/j.biochi.2019.03.008. [17] AJAMIAN L, ABEL K, RAO S, et al. HIV-1 recruits UPF1 but excludes UPF2 to promote nucleocytoplasmic export of the genomic RNA[J]. Biomolecules, 2015, 5( 4): 2808- 2839. DOI: 10.3390/biom5042808. [18] SAIKRUANG W, YAN PING L ANG, ABE H, et al. The RNA helicase DDX3 promotes IFNB transcription via enhancing IRF-3/p300 holo complex binding to the IFNB promoter[J]. Sci Rep, 2022, 12( 1): 3967. DOI: 10.1038/s41598-022-07876-z. [19] LI QS, PÈNE V, KRISHNAMURTHY S, et al. Hepatitis C virus infection activates an innate pathway involving IKK-α in lipogenesis and viral assembly[J]. Nat Med, 2013, 19( 6): 722- 729. DOI: 10.1038/nm.3190. [20] RYAN CS, SCHRÖDER M. The human DEAD-box helicase DDX3X as a regulator of mRNA translation[J]. Front Cell Dev Biol, 2022, 10: 1033684. DOI: 10.3389/fcell.2022.1033684. [21] HEATON SM, GORRY PR, BORG NA. DExD/H-box helicases in HIV-1 replication and their inhibition[J]. Trends Microbiol, 2023, 31( 4): 393- 404. DOI: 10.1016/j.tim.2022.11.001. [22] FOX D, MAN SM. DDX3X: Stressing the NLRP3 inflammasome[J]. Cell Res, 2019, 29( 12): 969- 970. DOI: 10.1038/s41422-019-0250-8. [23] LUO TT, YANG SZ, ZHAO TM, et al. Hepatocyte DDX3X protects against drug-induced acute liver injury via controlling stress granule formation and oxidative stress[J]. Cell Death Dis, 2023, 14( 7): 400. DOI: 10.1038/s41419-023-05913-x. [24] PAN ZZ, XU L, ZHANG XY, et al. Construction and identification of Ddx3x conditional liver knockout mouse model based on CRISPR/Cas9 combined with Cre-LoxP method[J]. Chin J Gastroenterol Hepatol, 2024, 33( 10): 1275- 1280. DOI: 10.3969/j.issn.1006-5709.2024.10.002.潘桢桢, 徐玲, 张向颖, 等. 基于CRISPR/Cas9联合Cre-LoxP方法的Ddx3x肝脏条件性敲除小鼠模型的构建与鉴定[J]. 胃肠病学和肝病学杂志, 2024, 33( 10): 1275- 1280. DOI: 10.3969/j.issn.1006-5709.2024.10.002. [25] SAMIR P, KESAVARDHANA S, PATMORE DM, et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome[J]. Nature, 2019, 573( 7775): 590- 594. DOI: 10.1038/s41586-019-1551-2. [26] KIM SY, KYAW YY, CHEONG J. Functional interaction of endoplasmic reticulum stress and hepatitis B virus in the pathogenesis of liver diseases[J]. World J Gastroenterol, 2017, 23( 43): 7657- 7665. DOI: 10.3748/wjg.v23.i43.7657. [27] SUBRAMANIAN K, PAUL S, LIBBY A, et al. HERV1-env induces unfolded protein response activation in autoimmune liver disease: A potential mechanism for regulatory T cell dysfunction[J]. J Immunol, 2023, 210( 6): 732- 744. DOI: 10.4049/jimmunol.2100186. [28] OYADOMARI S, MORI M. Roles of CHOP/GADD153 in endoplasmic reticulum stress[J]. Cell Death Differ, 2004, 11( 4): 381- 389. DOI: 10.1038/sj.cdd.4401373. [29] ADJIBADE P, GRENIER ST-SAUVEUR V, BERGEMAN J, et al. DDX3 regulates endoplasmic reticulum stress-induced ATF4 expression[J]. Sci Rep, 2017, 7( 1): 13832. DOI: 10.1038/s41598-017-14262-7. [30] HAN J, BACK SH, HUR J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death[J]. Nat Cell Biol, 2013, 15( 5): 481- 490. DOI: 10.1038/ncb2738. -



 PDF下载 ( 13813 KB)
PDF下载 ( 13813 KB)

 下载:
下载: