Numb基因过表达的人脐带间充质干细胞移植对胆汁淤积性肝纤维化的干预作用及其机制
DOI: 10.12449/JCH260110
Effect and mechanism of transplantation of human umbilical cord mesenchymal stem cells with overexpression of the Numb gene in treatment of cholestatic liver fibrosis
-
摘要:
目的 探讨Numb基因过表达的人脐带间充质干细胞(hUC-MSC)移植对胆汁淤积性肝纤维化(CLF)的干预作用及其机制。 方法 采用慢病毒转染技术过表达hUC-MSC的Numb基因(hUC-MSCNumb-OE),并以空载慢病毒转染的hUC-MSC(hUC-MSCOE-EV)为阴性对照。采用胆管结扎(BDL)建立CLF大鼠模型,随机分为BDL组、hUC-MSC组、hUC-MSCOE-EV组和hUC-MSCNumb-OE组,同时设有假手术组(Sham组)。各干预组于BDL后经脾脏一次性注射相应细胞,4周末取材。检测血清生化学、肝组织病理、肝组织羟脯氨酸(Hyp)水平、肝星状细胞活化、胆管反应、肝再生及Numb-p53信号轴关键分子的表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与BDL组比较,hUC-MSC组和hUC-MSCOE-EV组血清生化学指标(天冬氨酸氨基转移酶、γ-谷氨酰转移酶、总胆汁酸、总胆红素和直接胆红素)、肝纤维化相关指标(Hyp、α-平滑肌肌动蛋白、肿瘤坏死因子-α和转化生长因子-β1)、胆管反应指标(细胞角蛋白7、细胞角蛋白19)均显著降低,差异有统计学意义(P值均<0.05);且hUC-MSCNumb-OE组的上述指标较hUC-MSCOE-EV组进一步改善,差异有统计学意义(P值均<0.05)。此外,与hUC-MSCOE-EV组比较,hUC-MSCNumb-OE组肝再生相关指标(白蛋白、肝细胞核因子4α)、Numb-p53信号轴相关分子(Numb、pNumb、Mdm2、p53)的表达水平均较hUC-MSCOE-EV组显著改善,差异有统计学意义(P值均<0.05)。 结论 过表达Numb基因可增强hUC-MSC对CLF的干预效果,其机制可能与激活Numb-PTBL-p53-HNF4α轴,促hUC-MSC肝向分化,进而促进肝再生有关。 -
关键词:
- 肝纤维化 /
- Numb基因 /
- 脐带间充质干细胞 /
- 胆管反应 /
- Numb-PTBL-p53-HNF4α轴
Abstract:Objective To investigate the effect and mechanism of transplantation of human umbilical cord mesenchymal stem cell (hUC-MSC) with overexpression of the Numb gene in the treatment of cholestatic liver fibrosis (CLF). Methods The technique of lentiviral transfection was used to induce the overexpression of the Numb gene in hUC-MSC (hUC-MSCNumb-OE), and hUC-MSC transfected with empty vector (hUC-MSCOE-EV) was used as negative control. Bile duct ligation (BDL) was performed to establish a rat model of CLF, and then the rats were randomly divided into BDL group, hUC-MSC group, hUC-MSCOE-EV group, and hUC-MSCNumb-OE group, while a sham-operation group was also established. The rats in the intervention groups were given a single splenic injection of the corresponding cells after BDL, and samples were collected at the end of week 4. Related indicators were measured, including serum biochemistry, liver histopathology, the content of hydroxyproline (Hyp) in the liver, hepatic stellate cell activation, ductular reaction, liver regeneration, and the expression levels of key molecules in the Numb-p53 signaling axis. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the BDL group, the hUC-MSC group and the hUC-MSCOE-EV group had significant reductions in the levels of serum biochemical parameters (aspartate aminotransferase, gamma-glutamyl transpeptidase, total bile acid, total bilirubin, and direct bilirubin), liver fibrosis markers (the content of Hyp and the expression levels of alpha-smooth muscle actin, tumor necrosis factor-α, and transforming growth factor-beta 1), and ductular reaction markers (the expression levels of CK7 and CK19) (all P <0.05), and compared with the hUC-MSCOE-EV group, the hUC-MSCNumb-OE group had significantly greater improvements in the above indicators (all P <0.05). In addition, compared with the hUC-MSCOE-EV group, the hUC-MSCNumb-OE group had significant improvements in the expression levels of liver regeneration-related markers (albumin and hepatocyte nuclear factor 4α) and the molecules associated with the Numb-p53 signaling axis (Numb, pNumb, Mdm2, and p53) (all P <0.05). Conclusion Overexpression of the Numb gene can enhance the therapeutic effect of hUC-MSC on CLF, possibly by activating the Numb-PTBL-p53-HNF4α axis, promoting the hepatic differentiation of hUC-MSCs and subsequently enhancing liver regeneration. -
注: a,苏木素-伊红染色(×100);b,天狼星红胶原染色(×100);c,血清生化指标和肝组织Hyp水平。ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;ALP,碱性磷酸酶;GGT,γ-谷氨酰转移酶;TBA,总胆汁酸;TBil,总胆红素; DBil,直接胆红素; IBil,间接胆红素; Hyp,羟脯氨酸。*P<0.05,**P<0.01。
图 2 hUC-MSC Numb-OE移植对肝脏炎症反应及肝纤维化的影响
Figure 2. Effect of hUC-MSC Numb-OE transplantation on hepatic inflammation and fibrosis
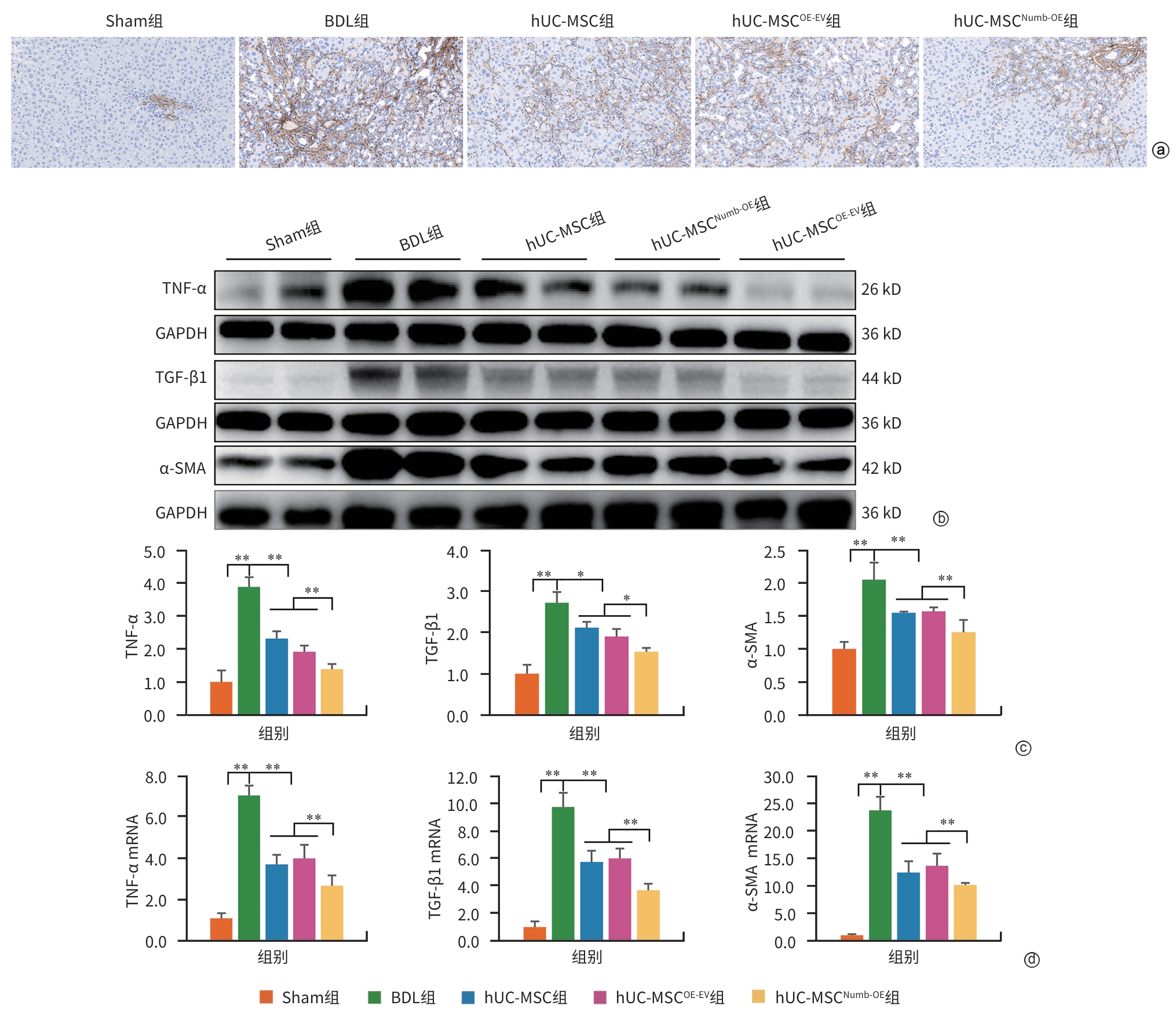
注: a,α-SMA免疫组化染色(×200);b,肝组织TNF-α、TGF-β1和 α-SMA免疫印迹条带;c,TNF-α、TGF-β1和 α-SMA免疫印迹条带灰度积分;d,TNF-α、TGF-β1和α-SMA的mRNA表达水平。TNF-α,肿瘤坏死因子-α;TGF-β1,转化生长因子-β1;α-SMA,α-平滑肌肌动蛋白;GAPDH,甘油醛-3-磷酸脱氢酶。*P<0.05,**P<0.01。
图 3 hUC-MSCNumb-OE移植对HSC活化的影响
Figure 3. Effect of hUC-MSCNumb-OE transplantation on hepatic stellate cell activation
注: a,Alb免疫组化染色(×200);b,HNF4α免疫组化染色(×200);c,Alb、HNF4α免疫印迹条带;d,Alb、HNF4α免疫印迹条带灰度积分;e,Alb、HNF4α mRNA表达水平;f,Numb1/2、pNumb、Mdm2和p53免疫印迹条带;g,Numb1/2、pNumb、Mdm2和p53免疫印迹条带灰度积分;h,Numb、Mdm2和p53的mRNA表达水平;i,血清Alb水平。Alb,白蛋白;HNF4α,肝细胞核因子4α;GAPDH,甘油醛-3-磷酸脱氢酶。*P<0.05,**P<0.01。
图 5 hUC-MSCNumb-OE移植对肝细胞增殖及Numb1/2-p53轴的影响
Figure 5. hUC-MSCNumb-OE transplantation on liver cell proliferation and Numb1/2-p53 axis
-
[1] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 3): 151- 166. DOI: 10.1038/s41575-020-00372-7. [2] FABRIS L, FIOROTTO R, SPIRLI C, et al. Pathobiology of inherited biliary diseases: A roadmap to understand acquired liver diseases[J]. Nat Rev Gastroenterol Hepatol, 2019, 16( 8): 497- 511. DOI: 10.1038/s41575-019-0156-4. [3] CORPECHOT C, CARRAT F, BONNAND AM, et al. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis[J]. Hepatology, 2000, 32( 6): 1196- 1199. DOI: 10.1053/jhep.2000.20240. [4] LIANG J, ZHANG HY, ZHAO C, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases[J]. Int J Rheum Dis, 2017, 20( 9): 1219- 1226. DOI: 10.1111/1756-185X.13015. [5] CHEN Z, KUANG QT, LAO XJ, et al. Differentiation of UC-MSCs into hepatocyte-like cells in partially hepatectomized model rats[J]. Exp Ther Med, 2016, 12( 3): 1775- 1779. DOI: 10.3892/etm.2016.3543. [6] DRISCOLL J, PATEL T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease[J]. J Gastroenterol, 2019, 54( 9): 763- 773. DOI: 10.1007/s00535-019-01599-1. [7] LIU PY, MAO YC, XIE Y, et al. Stem cells for treatment of liver fibrosis/cirrhosis: Clinical progress and therapeutic potential[J]. Stem Cell Res Ther, 2022, 13( 1): 356. DOI: 10.1186/s13287-022-03041-5. [8] COUTURIER L, VODOVAR N, SCHWEISGUTH F. Endocytosis by Numb breaks Notch symmetry at cytokinesis[J]. Nat Cell Biol, 2012, 14( 2): 131- 139. DOI: 10.1038/ncb2419. [9] SIDDIQUE HR, FELDMAN DE, CHEN CL, et al. NUMB phosphorylation destabilizes p53 and promotes self-renewal of tumor-initiating cells by a NANOG-dependent mechanism in liver cancer[J]. Hepatology, 2015, 62( 5): 1466- 1479. DOI: 10.1002/hep.27987. [10] COLALUCA IN, TOSONI D, NUCIFORO P, et al. NUMB controls p53 tumour suppressor activity[J]. Nature, 2008, 451( 7174): 76- 80. DOI: 10.1038/nature06412. [11] FILIPPONE MG, FREDDI S, ZECCHINI S, et al. Aberrant phosphorylation inactivates Numb in breast cancer causing expansion of the stem cell pool[J]. J Cell Biol, 2022, 221( 12): e202112001. DOI: 10.1083/jcb.202112001. [12] BALOGHOVA N, LIDAK T, CERMAK L. Ubiquitin ligases involved in the regulation of Wnt, TGF-β, and notch signaling pathways and their roles in mouse development and homeostasis[J]. Genes(Basel), 2019, 10( 10): 815. DOI: 10.3390/genes10100815. [13] ESPANOLA SG, SONG H, RYU E, et al. Haematopoietic stem cell-dependent Notch transcription is mediated by p53 through the Histone chaperone Supt16h[J]. Nat Cell Biol, 2020, 22( 12): 1411- 1422. DOI: 10.1038/s41556-020-00604-7. [14] XU YN, XU W, ZHANG X, et al. BM-MSCs overexpressing the Numb enhance the therapeutic effect on cholestatic liver fibrosis by inhibiting the ductular reaction[J]. Stem Cell Res Ther, 2023, 14( 1): 45. DOI: 10.1186/s13287-023-03276-w. [15] ZHANG X, DU GL, XU Y, et al. Inhibition of Notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes[J]. Lab Invest, 2016, 96( 3): 350- 360. DOI: 10.1038/labinvest.2015.149. [16] SHU YK, XU Q, XU YH, et al. Loss of Numb promotes hepatic progenitor expansion and intrahepatic cholangiocarcinoma by enhancing Notch signaling[J]. Cell Death Dis, 2021, 12( 11): 966. DOI: 10.1038/s41419-021-04263-w. [17] YU YB, SONG Y, CHEN Y, et al. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells[J]. Mol Med Rep, 2018, 18( 2): 2009- 2016. DOI: 10.3892/mmr.2018.9181. [18] SUK KT, YOON JH, KIM MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial[J]. Hepatology, 2016, 64( 6): 2185- 2197. DOI: 10.1002/hep.28693. [19] SHI M, LI YY, XU RN, et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial[J]. Hepatol Int, 2021, 15( 6): 1431- 1441. DOI: 10.1007/s12072-021-10199-2. [20] MOHAMADNEJAD M, ALIMOGHADDAM K, BAGHERI M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis[J]. Liver Int, 2013, 33( 10): 1490- 1496. DOI: 10.1111/liv.12228. [21] LANTHIER N, LIN-MARQ N, RUBBIA-BRANDT L, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: What is the impact on liver histology and gene expression patterns[J]. Stem Cell Res Ther, 2017, 8( 1): 88. DOI: 10.1186/s13287-017-0541-2. [22] WEI HY, LI FF, XUE TT, et al. microRNA-122-functionalized DNA tetrahedron stimulate hepatic differentiation of human mesenchymal stem cells for acute liver failure therapy[J]. Bioact Mater, 2023, 28: 50- 60. DOI: 10.1016/j.bioactmat.2023.04.024. [23] XU T, NI MM, XING-LI, et al. NLRC5 regulates TGF-β1-induced proliferation and activation of hepatic stellate cells during hepatic fibrosis[J]. Int J Biochem Cell Biol, 2016, 70: 92- 104. DOI: 10.1016/j.biocel.2015.11.010. [24] ZHANG K, ZHANG MX, MENG XX, et al. Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways[J]. Mil Med Res, 2023, 10( 1): 56. DOI: 10.1186/s40779-023-00494-4. [25] CHOI SS, DIEHL AM. Epithelial-to-mesenchymal transitions in the liver[J]. Hepatology, 2009, 50( 6): 2007- 2013. DOI: 10.1002/hep.23196. [26] NOVO E, di BONZO LV, CANNITO S, et al. Hepatic myofibroblasts: A heterogeneous population of multifunctional cells in liver fibrogenesis[J]. Int J Biochem Cell Biol, 2009, 41( 11): 2089- 2093. DOI: 10.1016/j.biocel.2009.03.010. [27] GUNEWARDENA S, HUCK I, WALESKY C, et al. Progressive loss of hepatocyte nuclear factor 4 alpha activity in chronic liver diseases in humans[J]. Hepatology, 2022, 76( 2): 372- 386. DOI: 10.1002/hep.32326. [28] XU YY, ZHU YD, HU SW, et al. Hepatocyte nuclear factor 4α prevents the steatosis-to-NASH progression by regulating p53 and bile acid signaling(in mice)[J]. Hepatology, 2021, 73( 6): 2251- 2265. DOI: 10.1002/hep.31604. [29] COLALUCA IN, BASILE A, FREIBURGER L, et al. A Numb-Mdm2 fuzzy complex reveals an isoform-specific involvement of Numb in breast cancer[J]. J Cell Biol, 2018, 217( 2): 745- 762. DOI: 10.1083/jcb.201709092. [30] SU DX, LI YX, ZHANG WJ, et al. SPTAN1/NUMB axis senses cell density to restrain cell growth and oncogenesis through Hippo signaling[J]. J Clin Invest, 2023, 133( 20): e168888. DOI: 10.1172/JCI168888. -



 PDF下载 ( 87365 KB)
PDF下载 ( 87365 KB)

 下载:
下载: