十二指肠乳头旁憩室对原发性胆总管结石患者胆汁微生态的影响
DOI: 10.12449/JCH250923
Influence of juxtapapillary duodenal diverticula on bile microecology in patients with primary common bile duct stones
-
摘要:
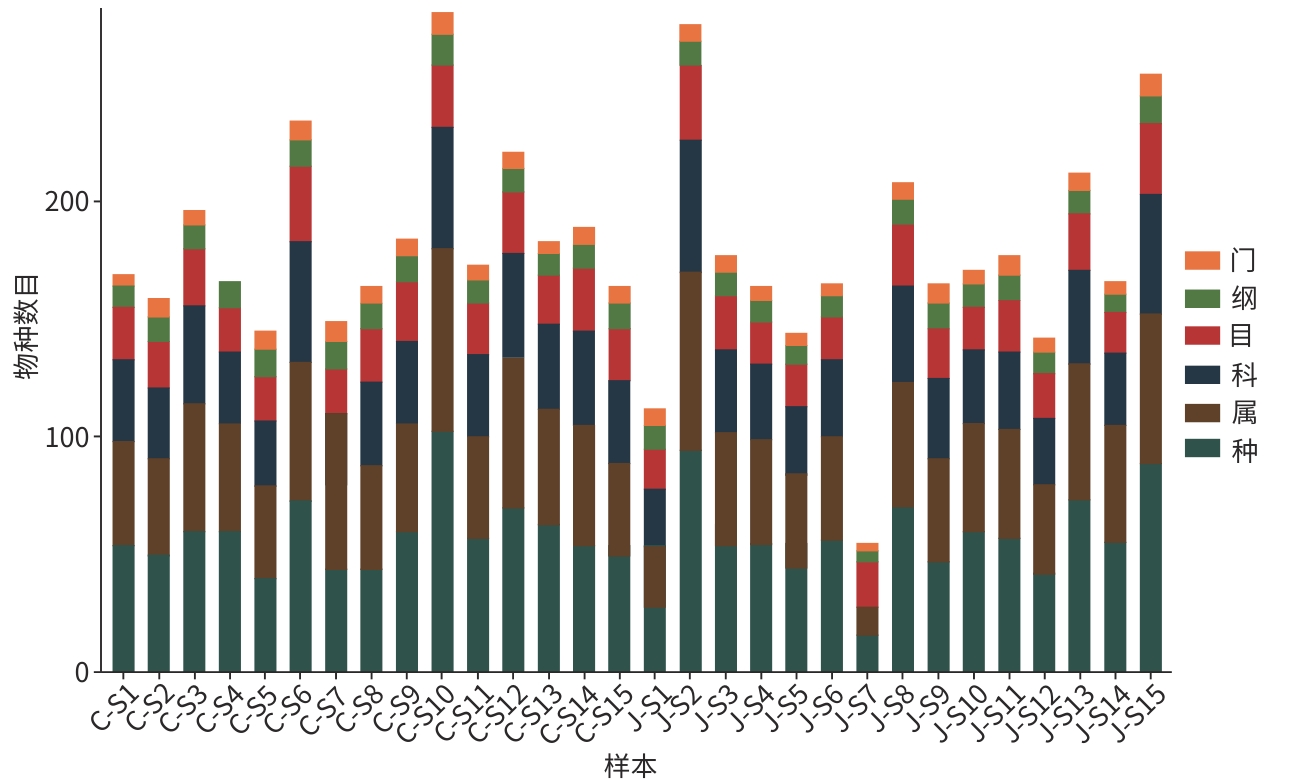
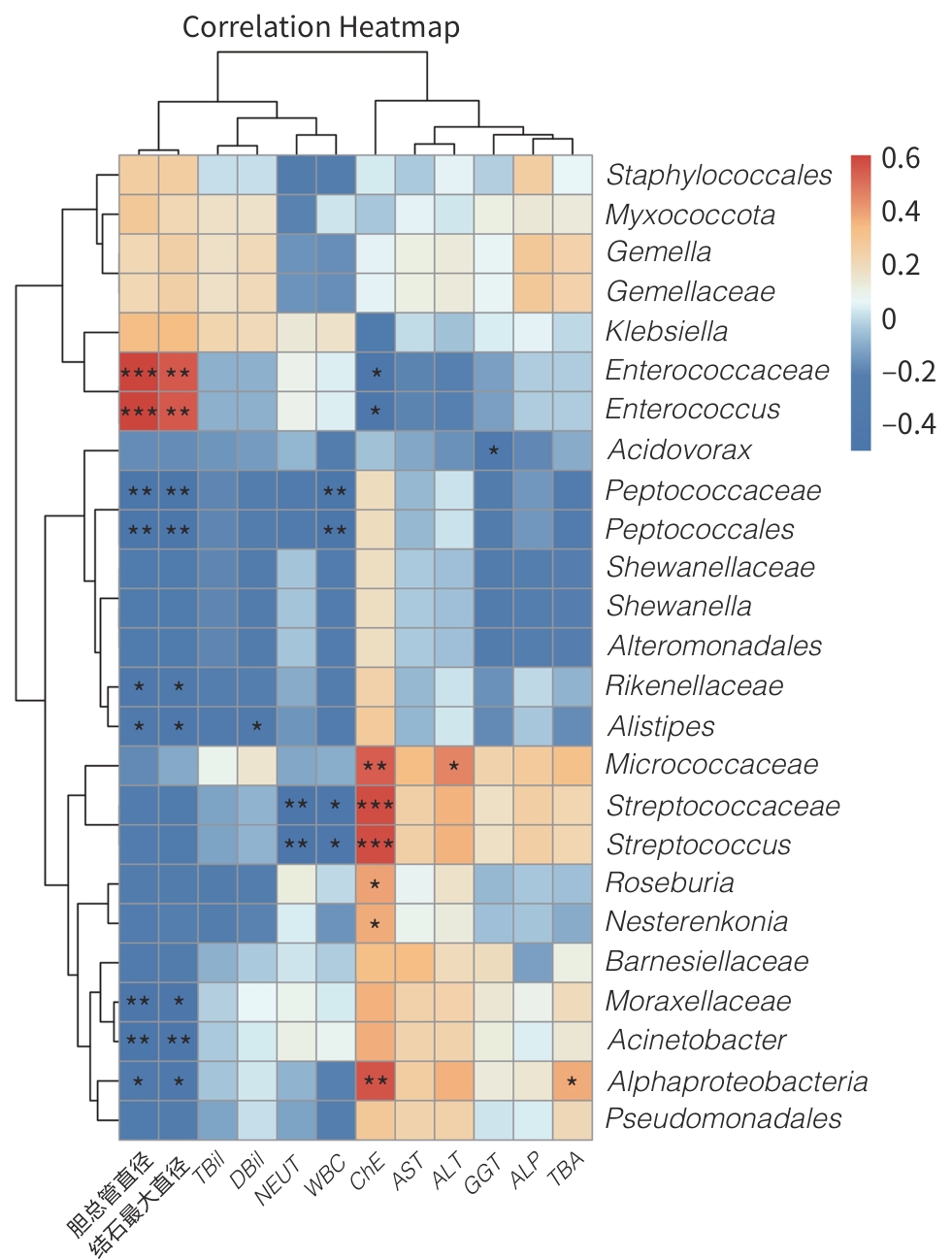
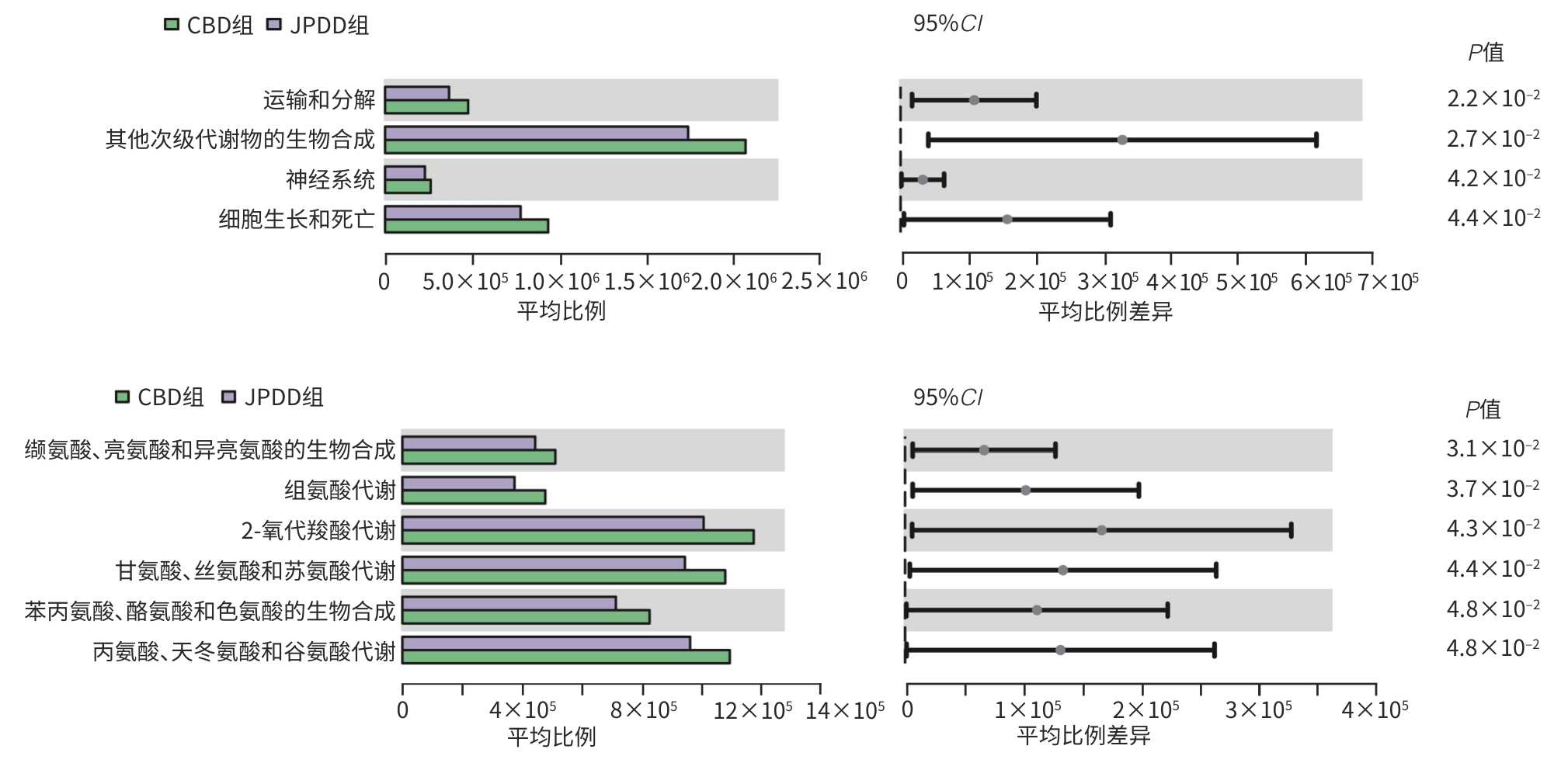
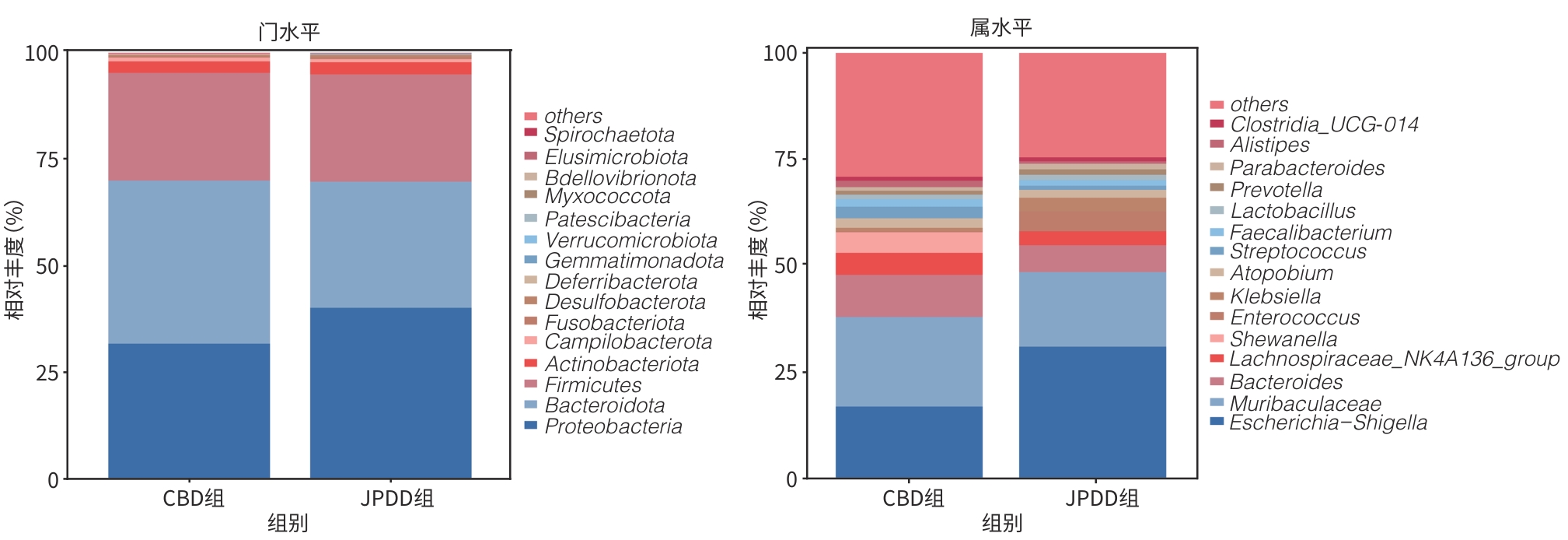
目的 探讨合并十二指肠乳头旁憩室(JPDD)的胆总管结石(CBD)及单纯胆总管结石患者的胆汁微生物特点。 方法 前瞻性纳入2024年1月—5月河北省人民医院消化内科收治的CBD患者30例,根据是否合并JPDD分为JPDD组(n=15)和CBD组(n=15)。在内镜逆行胆胰管造影术中收集胆汁,行16S rRNA微生物测序分析,比较两组微生物组成、多样性及代谢通路差异。符合正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U秩和检验。计数资料两组间比较采用χ2检验或Fisher精确检验法。临床指标与微生物物种丰度间的相关性采用Pearson相关分析或Spearman秩相关分析。 结果 JPDD组结石最大直径[(10.87±3.42) mm vs (6.80±2.08) mm, t=3.94, P<0.01)和胆总管直径[(14.73±3.95) mm vs (9.67±2.64) mm, t=4.13, P<0.01)均大于CBD组。胆汁微生物分析发现,两组最常见的菌门均为变形菌门、拟杆菌门、厚壁菌门、放线菌门,JPDD组中变形菌门占主导地位。在属和种水平,JPDD组大肠埃希氏-志贺氏菌属、肠球菌属、大肠杆菌相对丰度更高。两组Alpha多样性相似,Beta多样性差异明显(Adonis检验,P<0.05)。LEfSe分析发现,两组存在25个差异菌种(LDA>2),JPDD组肠杆菌属、肠球菌科、克雷伯菌属等7个菌群富集;消化球菌科、罗斯拜瑞氏菌属、别样杆菌属等18个菌群在CBD组中富集明显(P<0.05)。相关性分析发现,JPDD组显著富集的肠球菌科、肠球菌属与胆总管直径及结石最大直径呈正相关(P值均<0.01);而表达下降的消化球菌科、不动杆菌属、别样杆菌属等与胆总管直径及结石最大直径呈负相关(P值均<0.05)。胆汁微生物代谢富集通路发现,两组在细胞生长和死亡、运输和分解、神经系统,以及缬氨酸、亮氨酸和异亮氨酸的生物合成,组氨酸代谢等10条代谢通路有统计学差异(P值均<0.05)。 结论 JPDD的存在会导致胆汁微生物群发生改变(如肠球菌属升高、罗斯拜瑞氏菌属下降),特定菌群及其代谢可能促进CBD的形成。 -
关键词:
- 十二指肠乳头旁憩室 /
- 胆总管结石病 /
- 微生物群 /
- 高通量核苷酸序列分析
Abstract:Objective To investigate the microbiological characteristics of bile in patients with common bile duct stones alone or comorbid with juxtapapillary duodenal diverticula (JPDD). Methods A prospective study was conducted among 30 patients with common bile duct stones who were admitted to Department of Gastroenterology, Hebei General Hospital, from January to May 2024, and according to the presence or absence of JPDD, they were divided into JPDD group and simple common bile duct stones group (CBD group), with 15 patients in each group. Bile samples were collected during endoscopic retrograde cholangiopancreatography, and 16S rRNA microbial sequencing was performed to compare the differences in microbial composition, diversity, and metabolic pathways between the two groups. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U rank sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups. Pearson correlation analysis or Spearman correlation analysis was used to analyze the correlation between clinical indicators and microbial species abundance. Results Clinical data showed that compared with the CBD group, the JPDD group had significantly greater maximum diameter of stones (10.87±3.42 mm vs 6.80±2.08 mm, t=3.94, P<0.01) and common bile duct diameter (14.73±3.95 mm vs 9.67±2.64 mm, t=4.13, P<0.01). The microbiological analysis of the bile showed that Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria were the most common phyla in both groups, and Proteobacteria was the dominate phylum in the JPDD group. At the genus and species levels, the JPDD group had higher relative abundances of Escherichia-Shigella, Enterococcus, and Escherichia coli. Alpha diversity was similar between the two groups, and there was a significant difference in beta diversity between the two groups (Adonis test, P<0.05). The LEfSe analysis identified 25 differentially expressed species (LDA>2) between the two groups, and the JPDD group had enrichment of 7 flora such as Enterobacter, Enterococcaceae, and Klebsiella, while the CBD group had significant enrichment of 18 flora such as Peptococcaceae, Roseburia, and Alistipes (P<0.05). The correlation analysis showed that Enterococcaceae and Enterococcus significantly enriched in the JPDD group were positively correlated with the diameter of the common bile duct and the maximum diameter of stones (P<0.01), whereas Peptococcaceae, Acinetobacter, and Alistipes with reductions in expression were negatively correlated with the diameter of the common bile duct and the maximum diameter of stones (P<0.05). The enrichment analysis of biliary microbial metabolic pathways showed that there were significant differences between the two groups in 10 metabolic pathways such as cell growth and death, transportation and decomposition, nervous system, biosynthesis of valine, leucine, and isoleucine, and histidine metabolism (P<0.05). Conclusion The presence of JPDD may lead to alterations in bile microbiota, such as an increase in Enterococcus and a reduction in Roseburia, and specific flora and metabolism can promote the formation of common bile duct stones. -
表 1 患者一般临床资料分析
Table 1. Analysis of general clinical data of patients
指标 JPDD组(n=15) CBD组(n=15) 统计值 P值 性别[例(%)] 0.272 男 9(60.00) 5(33.33) 女 6(40.00) 10(66.67) 年龄(岁) 73.73±8.22 64.33±17.10 Z=-1.37 0.171 BMI(kg/m2) 24.25±3.44 26.78±4.45 t=-1.74 0.092 既往胆囊切除术[例(%)] 4(26.67) 6(40.00) 0.700 高血压[例(%)] 6(40.00) 7(46.67) 0.999 冠状动脉粥样硬化性心脏病[例(%)] 2(13.33) 3(20.00) 0.999 糖尿病[例(%)] 0(0.00) 2(13.33) 0.483 脑梗死[例(%)] 3(20.00) 1(6.67) 0.598 胆总管直径(mm) 14.73±3.95 9.67±2.64 t=4.13 <0.001 结石最大直径(mm) 10.87±3.42 6.80±2.08 t=3.94 <0.001 CBD数量[例(%)] 0.710 1个 8(53.33) 10(66.67) ≥2个 7(46.67) 5(33.33) WBC(×109/L) 9.50±3.53 7.14±1.84 t=2.30 0.032 NEUT(%) 79.83±10.46 79.38±11.01 t=0.12 0.909 TBil(μmol/L) 47.40(21.00~73.85) 31.30(13.00~67.55) Z=-0.94 0.345 DBil(μmol/L) 24.40(7.80~38.05) 12.70(2.75~39.95) Z=-1.02 0.309 ALT(U/L) 123.60(37.95~372.90) 154.40(33.70~364.50) Z=-0.08 0.935 ASL(U/L) 75.70(27.05~250.35) 129.70(28.25~306.25) Z=-0.12 0.902 GGT(U/L) 275.50(80.95~502.35) 147.10(54.40~423.85) Z=-0.53 0.595 ALP(U/L) 131.90(74.45~261.90) 121.10(92.75~159.70) Z=-0.20 0.838 ChE(U/L) 6 020.13±1 705.08 7 302.20±1 580.29 t=-2.14 0.042 TBA(U/L) 28.60(14.00~56.95) 79.89(3.05~134.74) Z=-0.37 0.713 胆管炎级别[例(%)] 0.299 不伴有胆管炎 6(40.00) 9(60.00) Ⅰ级 4(26.67) 5(33.33) Ⅱ级 5(33.33) 1(6.67) 术中插管困难[例(%)] 0(0.00) 2(13.33) 0.483 注:NEUT,中性粒细胞百分比;TBA,胆汁酸。
-
[1] LI SQ, GUIZZETTI L, MA C, et al. Epidemiology and outcomes of choledocholithiasis and cholangitis in the United States: Trends and urban-rural variations[J]. BMC Gastroenterol, 2023, 23( 1): 254. DOI: 10.1186/s12876-023-02868-3. [2] TAZUMA S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones(common bile duct and intrahepatic)[J]. Best Pract Res Clin Gastroenterol, 2006, 20( 6): 1075- 1083. DOI: 10.1016/j.bpg.2006.05.009. [3] SKAR V, SKAR AG, MIDTVEDT T, et al. Beta-glucuronidase-producing bacteria in bile from the common bile duct in patients treated with endoscopic papillotomy for gallstone disease[J]. Scand J Gastroenterol, 1986, 21( 2): 253- 256. DOI: 10.3109/00365528609034656. [4] STEWART L, PONCE R, OESTERLE AL, et al. Pigment gallstone pathogenesis: Slime production by biliary bacteria is more important than beta-glucuronidase production[J]. J Gastrointest Surg, 2000, 4( 5): 547- 553. DOI: 10.1016/s1091-255x(00)80100-6. [5] FENG R, ZHANG TY, KAYANI MUR, et al. Patients with primary and secondary bile duct stones harbor distinct biliary microbial composition and metabolic potential[J]. Front Cell Infect Microbiol, 2022, 12: 881489. DOI: 10.3389/fcimb.2022.881489. [6] KIM CW, CHANG JH, KIM JH, et al. Size and type of periampullary duodenal diverticula are associated with bile duct diameter and recurrence of bile duct stones[J]. J Gastroenterol Hepatol, 2013, 28( 5): 893- 898. DOI: 10.1111/jgh.12184. [7] KIRIYAMA S, KOZAKA K, TAKADA T, et al. Tokyo guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis(with videos)[J]. J Hepatobiliary Pancreat Sci, 2018, 25( 1): 17- 30. DOI: 10.1002/jhbp.512. [8] DEPOMMIER C, EVERARD A, DRUART C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study[J]. Nat Med, 2019, 25( 7): 1096- 1103. DOI: 10.1038/s41591-019-0495-2. [9] FERNANDES MR, AGGARWAL P, COSTA RGF, et al. Targeting the gut microbiota for cancer therapy[J]. Nat Rev Cancer, 2022, 22( 12): 703- 722. DOI: 10.1038/s41568-022-00513-x. [10] LIU Q, ZHENG LY, WANG Y, et al. Primary choledocholithiasis occurrence and recurrence is synergetcally modulated by the bile microbiome and metabolome alternations[J]. Life Sci, 2023, 331: 122073. DOI: 10.1016/j.lfs.2023.122073. [11] XIAO M, ZHOU YK, WANG ZF, et al. The dysregulation of biliary tract microflora is closely related to primary choledocholithiasis: A multicenter study[J]. Sci Rep, 2024, 14( 1): 9004. DOI: 10.1038/s41598-024-59737-6. [12] GROMSKI MA, GUTTA A, LEHMAN GA, et al. Microbiology of bile aspirates obtained at ERCP in patients with suspected acute cholangitis[J]. Endoscopy, 2022, 54( 11): 1045- 1052. DOI: 10.1055/a-1790-1314. [13] SHIN JH, TILLOTSON G, MACKENZIE TN, et al. Bacteroides and related species: The keystone taxa of the human gut microbiota[J]. Anaerobe, 2024, 85: 102819. DOI: 10.1016/j.anaerobe.2024.102819. [14] ZHENG X, YAN YJ, LI X, et al. Microbial characteristics of bile in gallstone patients: A comprehensive analysis of 9, 939 cases[J]. Front Microbiol, 2024, 15: 1481112. DOI: 10.3389/fmicb.2024.1481112. [15] LEE J, PARK JS, BAE J, et al. Bile microbiome in patients with recurrent common bile duct stones and correlation with the duodenal microbiome[J]. Life(Basel), 2022, 12( 10): 1540. DOI: 10.3390/life12101540. [16] LYU ZT, YU TT, ZHANG LC, et al. Analysis of the relationship between bile duct and duodenal microbiota reveals that potential dysbacteriosis is the main cause of primary common bile duct stones[J]. Synth Syst Biotechnol, 2021, 6( 4): 414- 428. DOI: 10.1016/j.synbio.2021.11.002. [17] LIU LX, ZHAO ZX, HOU XF, et al. Effect of sphincter of Oddi dysfunction on the abundance of biliary microbiota(biliary microecology) in patients with common bile duct stones[J]. Front Cell Infect Microbiol, 2022, 12: 1001441. DOI: 10.3389/fcimb.2022.1001441. [18] YANG Y, ZHAO ZY, WU SD, et al. Structural or functional abnormality of sphincter of oddi: An important factor for the recurrence of choledocholithiasis after endoscopic treatment[J]. Ann Med, 2025, 57( 1): 2440119. DOI: 10.1080/07853890.2024.2440119. [19] LEUNG JW, LIU YL, LEUNG PS, et al. Expression of bacterial beta-glucuronidase in human bile: An in vitro study[J]. Gastrointest Endosc, 2001, 54( 3): 346- 350. DOI: 10.1067/mge.2001.117546. [20] MAKI T. Pathogenesis of calcium bilirubinate gallstone: Role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation[J]. Ann Surg, 1966, 164( 1): 90- 100. DOI: 10.1097/0000-0658-196607000-00010. [21] MUSSA M, PÉREZ-CRESPO PMM, LOPEZ-CORTES LE, et al. Risk factors and predictive score for bacteremic biliary tract infections due to Enterococcus faecalis and Enterococcus faecium: A multicenter cohort study from the PROBAC project[J]. Microbiol Spectr, 2022, 10( 4): e0005122. DOI: 10.1128/spectrum.00051-22. [22] KRIAA A, BOURGIN M, POTIRON A, et al. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects[J]. J Lipid Res, 2019, 60( 2): 323- 332. DOI: 10.1194/jlr.R088989. [23] ZHAO ZY, YANG Y, WU SD, et al. Role of secretory mucins in the occurrence and development of cholelithiasis[J]. Biomolecules, 2024, 14( 6): 676. DOI: 10.3390/biom14060676. [24] MA WJ, WU ZR, YANG Q, et al. Biliary antibiotics irrigation for E. coli-induced chronic proliferative cholangitis and hepatolithiasis: A pathophysiological study in rabbits[J]. Clin Res Hepatol Gastroenterol, 2020, 44( 3): 356- 367. DOI: 10.1016/j.clinre.2019.07.008. [25] SEO B, JEON K, MOON S, et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice[J]. Cell Host Microbe, 2020, 27( 1): 25- 40. e 6. DOI: 10.1016/j.chom.2019.11.001. [26] IMHANN F, VICH VILA A, BONDER MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease[J]. Gut, 2018, 67( 1): 108- 119. DOI: 10.1136/gutjnl-2016-312135. [27] SHENG TJ, YAN SM, LAN WR, et al. Research progress on the impact of gut microbiota on inflammatory bowel disease[J]. Chin J Med Offic, 2023, 51( 9): 904- 907. DOI: 10.16680/j.1671-3826.2023.09.06.盛天骄, 闫思蒙, 兰威儒, 等. 肠道微生物对炎症性肠病影响研究进展[J]. 临床军医杂志, 2023, 51( 9): 904- 907. DOI: 10.16680/j.1671-3826.2023.09.06. [28] KEREN N, KONIKOFF FM, PAITAN Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients[J]. Environ Microbiol Rep, 2015, 7( 6): 874- 880. DOI: 10.1111/1758-2229.12319. [29] LI GF, YU TT, DU HM, et al. Effect of Clostridium butyricum on the formation of primary choledocholithiasis based on intestinal microbiome and metabolome analysis[J]. J Appl Microbiol, 2023, 134( 8): lxad170. DOI: 10.1093/jambio/lxad170. [30] MOLINERO N, RUIZ L, MILANI C, et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile[J]. Microbiome, 2019, 7( 1): 100. DOI: 10.1186/s40168-019-0712-8. [31] LI GH, HUANG SJ, LI X, et al. Response of gut microbiota to serum metabolome changes in intrahepatic cholestasis of pregnant patients[J]. World J Gastroenterol, 2020, 26( 46): 7338- 7351. DOI: 10.3748/wjg.v26.i46.7338. [32] MEN L, GU ZH, WANG EH, et al. Fufang muji granules ameliorate liver fibrosis by reducing oxidative stress and inflammation, inhibiting apoptosis, and modulating overall metabolism[J]. Metabolites, 2024, 14( 8): 446. DOI: 10.3390/metabo14080446. -



 PDF下载 ( 2937 KB)
PDF下载 ( 2937 KB)

 下载:
下载: