恩格列净联合多纳非尼或仑伐替尼在大鼠体内的药代动力学相互作用分析
DOI: 10.12449/JCH250921
Pharmacokinetic interactions between empagliflozin and donafenib/lenvatinib in rats
-
摘要:
目的 探究恩格列净与多纳非尼、仑伐替尼联合用药对各药物药代动力学(简称药动学)参数的影响,为临床联合用药提供参考。 方法 健康雄性SD大鼠48只,分为8组:恩格列净单用1组和2组、多纳非尼单用组、仑伐替尼单用组、多纳非尼预处理+恩格列净组、仑伐替尼预处理+恩格列净组、恩格列净预处理+多纳非尼组、恩格列净预处理+仑伐替尼组,每组6只。恩格列净给药剂量为2.5 mg/kg,多纳非尼给药剂量为40 mg/kg,仑伐替尼给药剂量为1.2 mg/kg。单用组连续7 d灌胃空白溶剂,第7天给予空白溶剂后分别给予单次恩格列净、单次多纳非尼和单次仑伐替尼;预处理组连续7 d灌胃给予预处理药物,第7天给予预处理药物后给予单次合用药物。于不同时间点采集血样,分离血浆测定各药物浓度。多纳非尼、仑伐替尼和恩格列净的血浆浓度采用经过验证的超高效液相色谱-串联质谱法测定,采用非房室模型计算药物的药动学参数[药-时曲线下面积(AUC)、达峰时间(Tmax)、峰浓度(Cmax)、消除半衰期(t1/2)]。正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验。 结果 与恩格列净单用组相比,多纳非尼预处理+恩格列净组恩格列净的AUC0-t 和AUC0-∞均增加(P值分别为0.011、0.008);与恩格列净单用组相比,仑伐替尼预处理+恩格列净组恩格列净的AUC无显著变化,Tmax略有提前(P=0.019)。与多纳非尼单用组相比,恩格列净预处理+多纳非尼组多纳非尼的AUC0-t 和AUC0-∞均增加(P值分别为0.027、0.025),Cmax也显著增加(P=0.015),CLz/F和Vz/F显著降低(P值分别为0.005、0.004);与仑伐替尼单用组相比,恩格列净预处理+仑伐替尼组仑伐替尼的t1/2缩短约5 h(P=0.002),AUC0-t 呈降低趋势,但差异无统计学意义(P>0.05)。 结论 恩格列净与多纳非尼联合使用可影响彼此药动学参数,使两种药物暴露量明显增加,两药合用时应注意监测疗效与不良反应;而恩格列净与仑伐替尼联合使用时,两者暴露量均无明显改变。 Abstract:Objective To investigate the influence of empagliflozin combined with donafenib or lenvatinib on the pharmacokinetic parameters of each drug, and to provide a reference for combined medication in clinical practice. Methods A total of 48 healthy male Sprague-Dawley rats were divided into 8 groups: empagliflozin group 1 and 2, donafenib group, lenvatinib group, donafenib pretreatment+empagliflozin group, lenvatinib pretreatment + empagliflozin group, empagliflozin pretreatment+donafenib group, and empagliflozin pretreatment+lenvatinib group, with 6 rats in each group. The doses of empagliflozin, donafenib, and lenvatinib were 2.5 mg/kg, 40 mg/kg, and 1.2 mg/kg, respectively. The rats in the empagliflozin group, donafenib group, and lenvatinib group were given a blank solvent by gavage for 7 consecutive days, followed by a single dose of empagliflozin, donafenib, or lenvatinib on day 7 after the administration of the blank solvent; the rats in the pretreatment groups were given the pretreatment drug by gavage for 7 consecutive days, followed by a single dose of drug combination on day 7 after administration of the pretreatment drug. Blood samples were collected at different time points, and plasma was separated to measure the concentration of each drug. A validated ultra-performance liquid chromatography-tandem mass spectrometry method was used to measure the plasma concentrations of donafenib, lenvatinib, and empagliflozin, and a non-compartmental model was used to calculate the main pharmacokinetic parameters of each drug (area under the plasma concentration-time curve [AUC], time to peak [Tmax], peak concentration [Cmax], and half-life time [t1/2]). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups. Results Compared with the empagliflozin group, the donafenib pretreatment+empagliflozin group had significant increases in the AUC0-t and AUC0-∞ of empagliflozin (P=0.011 and 0.008), while the lenvatinib pretreatment+empagliflozin group had no significant change in the AUC of empagliflozin, with a slightly shorter Tmax (P=0.019). Compared with the donafenib group, the empagliflozin pretreatment+donafenib group had significant increases in the AUC0-t and AUC0-∞ of donafenib (P=0.027 and 0.025), as well as a significant increase in Cmax (P=0.015) and significant reductions in CLz/F and Vz/F (P=0.005 and 0.004); compared with the lenvatinib group, the empagliflozin pretreatment+lenvatinib group had a reduction in the t1/2 of lenvatinib by approximately 5 hours (P=0.002), with a trend of reduction in AUC0-t (P>0.05). Conclusion Empagliflozin combined with donafenib may alter the pharmacokinetic parameters of both drugs, leading to a significant increase in the exposure levels of both drugs, and efficacy and adverse reactions should be monitored during co-administration. There are no significant changes in the exposure levels of empagliflozin and lenvatinib during co-administration. -
Key words:
- Empagliflozin /
- Donafenib /
- Lenvatinib /
- Pharmacokinetics /
- Drug Interactions
-
表 1 UPLC-MS/MS测定大鼠血浆中恩格列净浓度的精密度与准确度
Table 1. Precision and accuracy of empagliflozin in rat plasma was determined by UPLC-MS/MS
理论质量浓度
(ng/mL)日内(n=6) 日间(n=18) 实测质量浓度(ng/mL) RSD(%) 准确度(%) 实测质量浓度(ng/mL) RSD(%) 准确度(%) 5 4.86±0.17 3.5 97.3 5.03±0.31 6.1 100.6 15 15.03±0.56 3.7 100.6 15.03±1.00 6.6 100.2 800 819.33±21.13 2.6 102.4 825.06±40.87 5.0 103.1 1 500 1 486.67±96.47 6.5 99.1 1 503.00±97.80 6.5 100.2 表 2 UPLC-MS/MS法测定大鼠血浆中恩格列净的基质效应
Table 2. Matrix effect of empagliflozin in rat plasma was determined by UPLC-MS/MS
理论质量浓度(ng/mL) 内标归一化基质效应 均值(%) RSD(%) 15 106.62±4.66 4.4 800 110.78±2.0 1.8 1 500 95.91±2.39 2.5 表 3 不同条件下恩格列净在大鼠血浆中的稳定性
Table 3. Stability of empagliflozin in rat plasma under different conditions
理论质量浓度(ng/mL) 实际测量质量浓度(ng/mL) RSD(%) 准确度(%) 处理后样品在自动进样器(15 ℃)放置6 h 15 15.17±1.07 7.1 101.1 800 830.00±40.65 4.9 103.8 1 500 1 478.33±93.06 6.6 98.6 室温(25 ℃±2 ℃)下放置4 h 15 15.83±0.94 5.9 105.6 800 844.17±29.67 3.5 105.5 1 500 1 473.33±95.64 6.5 98.2 -80 ℃放置30 d 15 14.88±0.77 5.2 99.2 800 848.67±37.74 4.5 106.1 1 500 1 521.67±113.74 7.8 101.4 -80 ℃至室温冻融3次 15 16.10±0.47 2.9 107.3 800 833.17±38.43 4.6 104.2 1 500 1 535.00±105.59 6.9 102.3 表 4 恩格列净单用和联合多纳非尼在大鼠体内的药动学参数
Table 4. Pharmacokinetic parameters of empagliflozin in rats after oral administration alone and combined multiple doses of donafenib
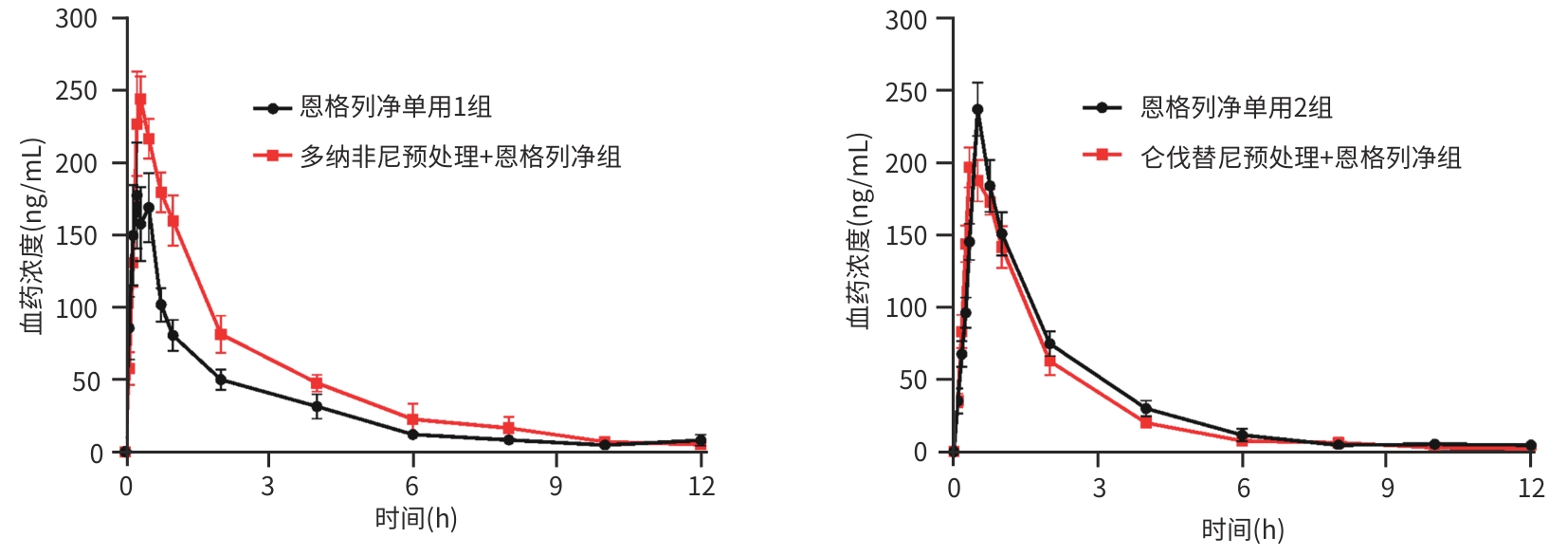
参数 恩格列净单用1组(n=6) 多纳非尼预处理+恩格列净组(n=6) 统计值 P值 AUC0-t (μg·h/L) 363.34±100.12 572.29±132.02 t=-3.089 0.011 AUC0-∞ (μg·h/L) 381.56±108.86 604.92±125.62 t=-3.291 0.008 Cmax (μg/L) 208.00(134.50~266.75) 223.50(218.00~312.25) Z=-1.123 0.261 Tmax (h) 0.38(0.25~0.56) 0.33(0.25~0.33) Z=-0.509 0.611 t1/2 (h) 3.24±1.10 4.51±3.34 t=-0.880 0.399 CLz/F (L·h-1·kg-1) 7.12±2.47 4.28±0.84 t=2.669 0.024 Vz/F (L/kg) 31.57±10.71 28.63±21.35 t=0.302 0.769 注:AUC,药-时曲线下面积;Cmax,最大血药浓度;Tmax,达峰时间;t1/2,消除半衰期;CLz/F,清除率与生物利用度比值;Vz/F,表观分布容积与生物利用度比值。
表 5 恩格列净单用和联合仑伐替尼在大鼠体内的药动学参数
Table 5. Pharmacokinetic parameters of empagliflozin in rats after oral administration alone and combined multiple doses of donafenib
参数 恩格列净单用2组(n=6) 仑伐替尼预处理+恩格列净组(n=6) 统计值 P值 AUC0-t (μg·h/L) 440.13±68.38 383.47±89.98 t=1.242 0.242 AUC0-∞ (μg·h/L) 458.15±78.27 397.31±99.18 t=1.180 0.265 Cmax (μg/L) 237.17±45.16 204.50±30.59 t=1.146 0.173 Tmax (h) 0.50(0.50~0.50) 0.33(0.33~0.50) Z=-2.345 0.019 t1/2 (h) 3.21±1.65 2.77±0.88 t=0.587 0.570 CLz/F(L·h-1·kg-1) 5.60±1.02 6.67±1.85 t=-1.242 0.243 Vz/F(L/kg) 24.51±9.56 26.34±11.00 t=-0.309 0.764 表 6 多纳非尼单用和联合恩格列净在大鼠体内的药动学参数
Table 6. Pharmacokinetic parameters of donafenib in rats after oral administration alone and combined multiple doses of empagliflozin
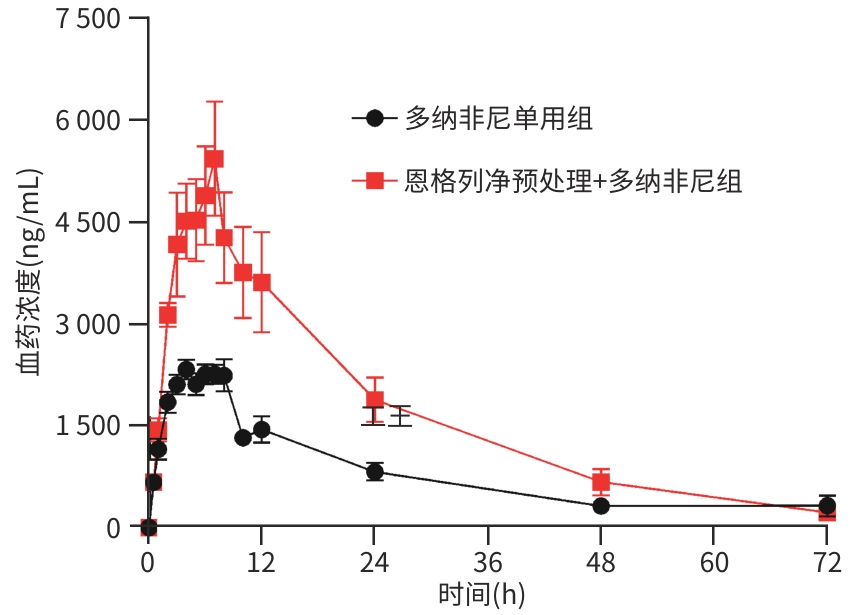
参数 多纳非尼单用组(n=6) 恩格列净预处理+多纳非尼组(n=6) t值 P值 AUC0-t (μg·h/L) 56 710.73±17 771.00 120 252.80±50 815.76 -2.891 0.027 AUC0-∞ (μg·h/L) 59 308.43±19 974.03 125 442.30±51 787.97 -2.918 0.025 Cmax (μg/L) 2 558.33±319.28 5 640.00±2 086.32 -3.576 0.015 Tmax (h) 6.17±1.72 7.00±0.63 -1.112 0.306 t1/2 (h) 15.32±2.38 15.85±2.63 -0.366 0.722 CLz/F (L·h-1·kg-1) 0.74±0.22 0.36±0.12 3.609 0.005 Vz/F (L/kg) 15.72±3.22 8.38±3.53 3.764 0.004 表 7 仑伐替尼单用和联合恩格列净在大鼠体内的药动学参数
Table 7. Pharmacokinetic parameters of lenvatinib in rats after oral administration alone and combined multiple doses of empagliflozin
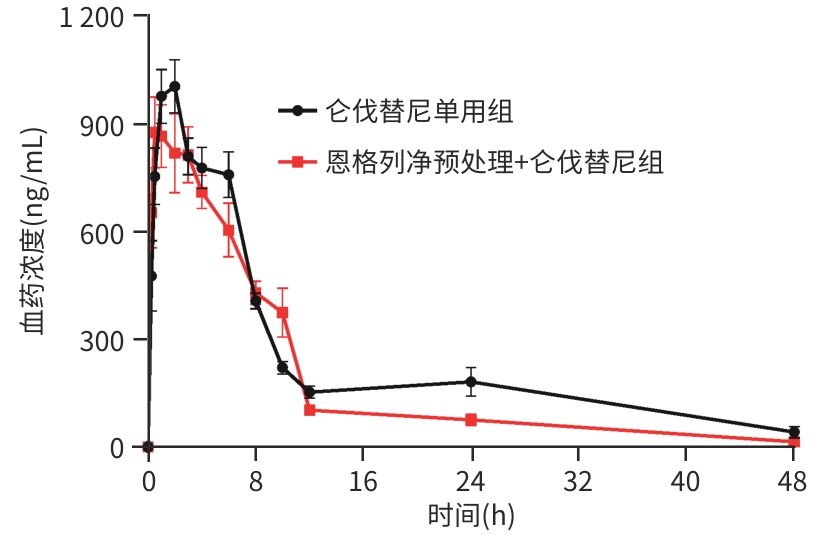
参数 仑伐替尼单用组(n=6) 恩格列净预处理+仑伐替尼组(n=6) 统计值 P值 AUC0-t (μg·h/L) 11 727.32±1487.50 9 536.09±2396.86 t=1.903 0.092 AUC0-∞ (μg·h/L) 12 250.02±1687.97 9 627.33±2399.64 t=2.190 0.053 Cmax (μg/L) 1 039.50±171.30 955.67±211.38 t=0.755 0.468 Tmax (h) 2.00(0.88~2.00) 1.50(0.50~2.50) Z=-0.259 0.796 t1/2 (h) 12.58±2.74 7.14±1.82 t=4.069 0.002 CLz/F (L·h-1·kg-1) 0.10±0.02 0.13±0.03 t=-2.166 0.068 Vz/F (L/kg) 1.68(1.63~1.92) 1.20(0.96~1.79) Z=-1.922 0.055 -
[1] SCOTT LJ. Lenvatinib: First global approval[J]. Drugs, 2015, 75( 5): 553- 560. DOI: 10.1007/s40265-015-0383-0. [2] GUPTA A, JARZAB B, CAPDEVILA J, et al. Population pharmacokinetic analysis of lenvatinib in healthy subjects and patients with cancer[J]. Br J Clin Pharmacol, 2016, 81( 6): 1124- 1133. DOI: 10.1111/bcp.12907. [3] HE XR, LI Y, LI YJ, et al. In vivo assessment of the pharmacokinetic interactions between donafenib and dapagliflozin, donafenib and canagliflozin in rats[J]. Biomed Pharmacother, 2023, 162: 114663. DOI: 10.1016/j.biopha.2023.114663. [4] MIRARCHI L, AMODEO S, CITARRELLA R, et al. SGLT2 inhibitors as the most promising influencers on the outcome of non-alcoholic fatty liver disease[J]. Int J Mol Sci, 2022, 23( 7): 3668. DOI: 10.3390/ijms23073668. [5] LIU ZH, DU WY, GUO CH, et al. Research progress of empagliflozin in the treatment of type 2 diabe-tes mellitus and cardiovascular and renal benefits[J]. Chin J Clin Pharmacol Ther, 2025, 30( 3): 412- 418. DOI: 10.12092/j.issn.1009-2501.2025.03.015.刘子涵, 杜文雨, 郭彩会, 等. 恩格列净治疗2型糖尿病和心肾获益的研究进展[J]. 中国临床药理学与治疗学, 2025, 30( 3): 412- 418. DOI: 10.12092/j.issn.1009-2501.2025.03.015. [6] STÖLLBERGER C, FINSTERER J, SCHNEIDER B. Adverse events and drug-drug interactions of sodium glucose co-transporter 2 inhibitors in patients treated for heart failure[J]. Expert Rev Cardiovasc Ther, 2023, 21( 11): 803- 816. DOI: 10.1080/14779072.2023.2273900. [7] CUI YJ, LI Y, FAN LJ, et al. UPLC-MS/MS method for the determination of Lenvatinib in rat plasma and its application to drug-drug interaction studies[J]. J Pharm Biomed Anal, 2021, 206: 114360. DOI: 10.1016/j.jpba.2021.114360. [8] Chinese Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China(PRC)-part IV(2020 edition)[M]. Beijing: China Medical Science Press, 2020.国家药典委员会. 中华人民共和国药典-四部(2020年版)[M]. 北京: 中国医药科技出版社, 2020. [9] SCHEEN AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor[J]. Clin Pharmacokinet, 2014, 53( 3): 213- 225. DOI: 10.1007/s40262-013-0126-x. [10] GARCIA-ROPERO A, BADIMON JJ, SANTOS-GALLEGO CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: The latest developments[J]. Expert Opin Drug Metab Toxicol, 2018, 14( 12): 1287- 1302. DOI: 10.1080/17425255.2018.1551877. [11] KEAM SJ, DUGGAN S. Donafenib: First approval[J]. Drugs, 2021, 81( 16): 1915- 1920. DOI: 10.1007/s40265-021-01603-0. [12] REAGAN-SHAW S, NIHAL M, AHMAD N. Dose translation from animal to human studies revisited[J]. FASEB J, 2008, 22( 3): 659- 661. DOI: 10.1096/fj.07-9574LSF. [13] MACHA S, KOENEN R, SENNEWALD R, et al. Effect of gemfibrozil, rifampicin, or probenecid on the pharmacokinetics of the SGLT2 inhibitor empagliflozin in healthy volunteers[J]. Clin Ther, 2014, 36( 2): 280- 290. e 1. DOI: 10.1016/j.clinthera.2014.01.003. [14] DENG YR, CAO GX, YAN B, et al. Effect of sorafenib and donafenib on the pharmacokinetics of ertugliflozin in rats[J]. J Clin Hepatol, 2025, 41( 1): 92- 98. DOI: 10.12449/JCH250114.邓艳茹, 曹格溪, 闫彬, 等. 索拉非尼和多纳非尼对大鼠体内艾托格列净药代动力学的影响[J]. 临床肝胆病杂志, 2025, 41( 1): 92- 98. DOI: 10.12449/JCH250114. [15] WANG XM, ZHANG X, HUANG XH, et al. The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4[J]. Xenobiotica, 2016, 46( 7): 651- 658. DOI: 10.3109/00498254.2015.1109160. [16] LI JM, WANG XQ, NING C, et al. Influences of ABC transporter and CYP3A4/5 genetic polymorphisms on the pharmacokinetics of lenvatinib in Chinese healthy subjects[J]. Eur J Clin Pharmacol, 2020, 76( 8): 1125- 1133. DOI: 10.1007/s00228-020-02879-z. [17] SHUMAKER R, ALURI J, FAN J, et al. Effects of ketoconazole on the pharmacokinetics of lenvatinib(E7080) in healthy participants[J]. Clin Pharmacol Drug Dev, 2015, 4( 2): 155- 160. DOI: 10.1002/cpdd.140. [18] SHUMAKER RC, ALURI J, FAN J, et al. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults[J]. Clin Drug Investig, 2014, 34( 9): 651- 659. DOI: 10.1007/s40261-014-0217-y. -



 PDF下载 ( 1792 KB)
PDF下载 ( 1792 KB)

 下载:
下载: