盐酸可洛派韦和索磷布韦联合或不联合利巴韦林治疗慢性HCV感染者的效果和安全性分析
DOI: 10.12449/JCH250912
Efficacy and safety of coblopasvir hydrochloride capsules/sofosbuvir tablets with or without ribavirin tablets in treatment of patients with chronic hepatitis C virus infection
-
摘要:
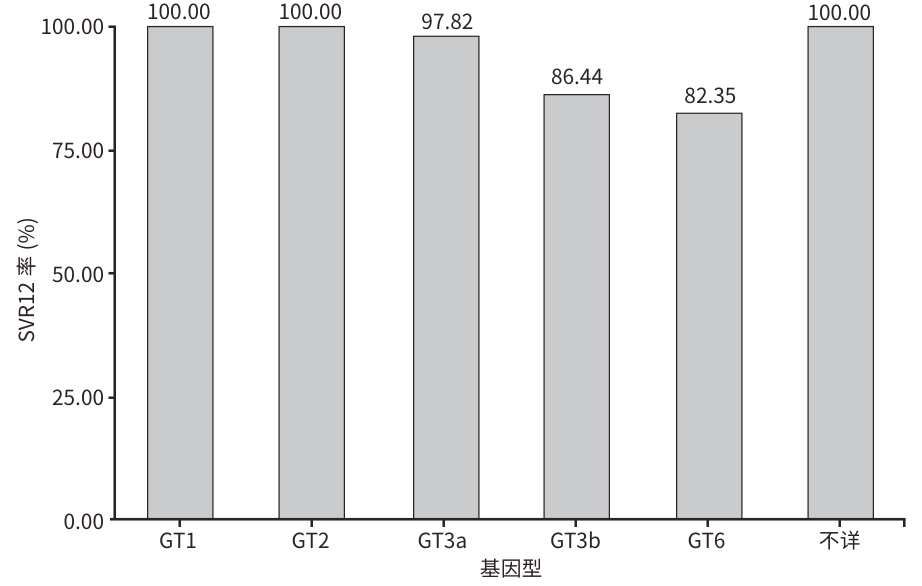
目的 评估真实世界中以盐酸可洛派韦和索磷布韦为基础的治疗方案对慢性HCV感染者的治疗效果、影响因素及安全性。 方法 选取2021年9月—2024年5月在昆明市第三人民医院就诊的253例患者,其中丙型肝炎代偿期肝硬化患者86例(CLC组),慢性丙型肝炎患者167例(CHC组)。使用盐酸可洛派韦(60 mg)和索磷布韦(400 mg)联合或不联合利巴韦林片治疗12周,停药后随访12周。主要评估治疗结束后12周持续病毒学应答(SVR12)率和安全性,其次评估治疗4周、治疗12周、停药12周时肝功能、肾功能、血常规、肝硬度值(LSM)的变化。计量资料两组间比较采用成组t检验和Mann-Whitney U检验;多组间比较采用Friedman检验,组内两两比较采用Bonferroni法。计数资料两组间比较采用χ2检验。采用Logistic回归分析SVR的影响因素。 结果 253例慢性HCV感染者的平均年龄(49.38±8.65)岁,男151例(59.7%),33.99%(n=86)合并肝硬化,25.69%(n=65)合并高血压,10.67%(n=27)合并HIV,8.70%(n=22)合并糖尿病,3.95%(n=10)合并肝细胞癌,1.98%(n=5)合并慢性乙型肝炎,7.91%(n=20)为经治患者。基因型分布:1型2.77%(n=7)、2型12.65%(n=32)、3型66.01%(n=167)、6型16.60%(n=42)、基因型不详1.98%(n=5)。患者总体SVR12率为92.09%,CLC组SVR12率为93.02%,CHC组SVR12率为91.02%。多因素Logistic分析结果显示,年龄(OR=1.086,95%CI:1.007~1.170,P=0.032)、HCC(OR=9.178,95%CI:1.722~48.912,P=0.009)是SVR的独立影响因素。与基线相比,治疗12周后CLC组ALT(χ2=107.103)、AST(χ2=90.602)、LSM(χ2=42.235),CHC组TBil(χ2=15.113)、ALT(χ2=202.237)、AST(χ2=161.193)、LSM(χ2=37.606)水平均下降,差异均有统计学意义(P值均<0.05)。严重不良反应事件发生率为1.58%,均未停药,积极对症处理后缓解。全部不良事件的发生率为23.72%,乏力(17.39%)和恶心(2.37%)最常见,在2周内消失或对症处理后可逐渐缓解。 结论 盐酸可洛派韦和索磷布韦联合或不联合利巴韦林治疗慢性HCV感染有较好的效果和安全性。 Abstract:Objective To investigate the therapeutic efficacy, influencing factors, and safety of a treatment regimen based on coblopasvir hydrochloride capsules/sofosbuvir tablets in patients with chronic hepatitis C virus (HCV) infection in a real-world setting. Methods A total of 253 patients who attended The Third People’s Hospital of Kunming from September 1, 2021 to May 31, 2024 were enrolled, among whom there were 86 patients with compensated liver cirrhosis (CLC group) and 167 patients with chronic hepatitis C (CHC group). The patients were treated with coblopasvir hydrochloride capsules (60 mg)/sofosbuvir tablets (400 mg) with or without ribavirin tablets for 12 weeks, and they were followed up for 12 weeks after drug withdrawal. The primary outcome measures were the rate of sustained virologic response at week 12 after treatment (SVR12) and safety, and the secondary outcome measures were the changes in liver function, renal function, blood routine, and liver stiffness measurements (LSM) after 4 weeks of treatment, after 12 weeks of treatment, and at 12 weeks after drug withdrawal. The independent-samples t test and the Mann-Whitney U test were used for comparison of continuous data between two groups, and the Friedman test was used for comparison between multiple groups, while the Bonferroni method was used for paired comparison within each group; the chi-square test was used for comparison of categorical data between two groups. The Logistic analysis was used to investigate related influencing factors. Results The 253 patients with chronic HCV infection had a mean age of 49.38±8.65 years, and there were 151 male patients (59.7%). Of all patients, 33.99% (86/253) had liver cirrhosis, 25.69% (65/253) had hypertension, 10.67% (27/253) had HIV infection, 8.70% (22/253) had diabetes, 3.95% (10/253) had liver cancer, 1.98% (5/253) had chronic hepatitis B, and 7.91% (20/253) were treatment-experienced patients. As for genotype distribution, 2.77% (7/253) had genotype 1, 12.65% (32/253) had genotype 2, 66.01% (167/253) had genotype 3, 16.60% (42/253) had genotype 6, and 1.98% (5/253) had unknown genotype. The patients had an overall SVR12 rate of 92.09%, with an SVR12 rate of 93.02% in the CLC group and 91.02% in the CHC group. The multivariate logistic regression analysis showed that age (odds ratio [OR]=1.086, 95% confidence interval [CI]: 1.007 — 1.170, P=0.032) and HCC (OR=9.178, 95%CI: 1.722 — 48.912, P=0.009) were independent influencing factors for sustained virologic response. Compared with baseline data, the CLC group had significant reductions in alanine aminotransferase (ALT) (χ2=107.103, P<0.05), aspartate aminotransferase (AST) (χ2=90.602, P<0.05), and LSM (χ2=42.235, P<0.05) after 12 weeks of treatment, while the CHC group had significant reductions in total bilirubin (χ2=15.113, P<0.05), ALT (χ2=202.237, P<0.05), AST (χ2=161.193, P<0.05), and LSM (χ2=37.606, P<0.05). The incidence rate of serious adverse events was 1.58%, and none of the patients withdrew from drug therapy; the patients with such events were relieved after active symptomatic treatment. The incidence rate of all adverse events was 23.72%, among which fatigue (17.39%) and nausea (2.37%) were the most common adverse events, and these events often disappeared within 2 weeks or were gradually relieved after symptomatic treatment. Conclusion Coblopasvir hydrochloride capsules/sofosbuvir tablets with or without ribavirin tablets has good efficacy and safety in the treatment of chronic HCV infection. -
Key words:
- Hepatitis C, Chronic /
- Coblopasvir Hydrochloride /
- Sofosbuvir /
- Ribavirin /
- Treatment Outcome
-
表 1 253例患者基线资料
Table 1. Baseline data of 253 patients
项目 合计(n=253) CHC组(n=167) CLC组(n=86) 统计值 P值 男/女(例) 151/102 91/76 60/26 χ2=5.506 0.019 年龄(岁) 49.38±8.65 47.87±8.75 52.31±7.68 t=-3.987 <0.001 高血压(否/是,例) 188/65 131/36 57/29 χ2=4.400 0.036 糖尿病(否/是,例) 231/22 158/9 73/13 χ2=6.765 0.009 HIV感染(否/是,例) 226/27 148/19 78/8 χ2=0.256 0.613 HBV感染(否/是,例) 248/5 166/1 82/4 χ2=2.948 0.086 HCC(否/是,例) 243/10 165/1 77/9 χ2=14.556 <0.001 治疗史(初治/经治,例) 233/20 155/12 78/8 χ2=0.349 0.554 利巴韦林(未联合/联合,例) 112/141 84/83 28/58 χ2=7.242 0.007 基因型(1/2/3/6/不详,例) 7/32/167/42/5 5/21/105/34/2 2/11/62/8/3 χ2=12.393 0.300 HCV RNA(log10 IU/mL) 5.97(5.11~6.56) 6.08(5.18~6.65) 5.79(4.95~6.26) Z=-2.038 0.042 TBil(μmol/L) 14.90(11.15~20.20) 13.50(10.00~17.60) 18.10(13.10~26.65) Z=-5.188 <0.001 ALT(U/L) 56(35~100) 51(32~93) 72(42~114) Z=-2.678 0.007 AST(U/L) 51(33~87) 40(28~71) 68(48~100) Z=-5.289 <0.001 PLT(×109/L) 175(117~225) 191(139~235) 123(78~184) Z=-6.229 <0.001 Hb(g/L) 151±23 153±19 148±29 t=1.387 0.168 WBC(×109/L) 5.24(4.29~6.43) 5.46(4.41~6.55) 5.03(3.62~6.17) Z=-2.542 0.011 Cr(μmol/L) 59(49~71) 60(50~71) 58(48~75) Z=-0.652 0.514 尿酸(μmol/L) 339±92 341±91 335±94 t=0.482 0.631 LSM(kPa) 11.70(7.85~17.99) 9.80(6.70~14.40) 15.95(11.27~27.82) Z=-6.404 <0.001 表 2 单因素和多因素Logistic回归分析
Table 2. Single factor and multifactor Logistic analysis
项目 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 女性 0.782(0.301~2.033) 0.614 年龄(岁) 1.101(1.035~1.171) 0.002 1.086(1.007~1.170) 0.032 肝硬化(否/是) 1.050(0.403~2.736) 0.921 HCC(否/是) 15.200(3.959~58.351) <0.001 9.178(1.722~48.912) 0.009 治疗史(初治/经治) 3.391(1.014~11.343) 0.048 利巴韦林(未联合/联合) 1.523(0.587~3.957) 0.387 饮酒史(否/是) 3.456(1.121~10.651) 0.031 糖尿病(否/是) 4.235(1.373~13.060) 0.012 高血压(否/是) 2.058(0.802~5.286) 0.134 HIV感染(否/是) 1.537(0.420~5.628) 0.516 WBC(×109/L) 0.978(0.771~1.242) 0.858 PLT(×109/L) 0.994(0.987~1.001) 0.087 Hb(g/L) 0.992(0.973~1.011) 0.420 TBil(μmol/L) 0.971(0.915~1.031) 0.337 ALT(U/L) 1.002(0.995~1.008) 0.647 GGT(U/L) 1.000(0.998~1.003) 0.824 Cr(μmol/L) 1.006(0.993~1.020) 0.346 HCV RNA(log10 IU/mL) 1.408(0.908~2.184) 0.127 LSM(kPa) 1.010(0.972~1.050) 0.610 AFP(ng/mL) 1.000(0.999~1.001) 0.855 表 3 20例未取得SVR12患者特征
Table 3. 20 cases without SVR12 patient characteristics obtained
编号 性别 年龄(岁) 基因型 诊断 复治 联合治疗 吸毒史 1 男 54 3b CHC 否 利巴韦林 有 2 女 57 6n CHC 否 无 无 3 男 46 6n CHC 否 无 有 4 男 50 3b CHC 否 利巴韦林 有 5 女 60 6n CHC 否 无 无 6 男 49 6n CHC 否 无 无 7 女 65 3a CLC、HCC 否 利巴韦林 无 8 女 59 6n CHC 否 无 有 9 女 55 3b CLC、HCC 是 利巴韦林 有 10 女 49 3b CHC、HIV感染 否 无 有 11 男 43 3b CHC、HIV感染 否 无 有 12 男 68 3a CHC 否 利巴韦林 不详 13 男 49 3b CHC、HIV感染 否 利巴韦林 有 14 女 63 3b CLC、HCC 否 利巴韦林 有 15 女 47 6n CHC 是 利巴韦林 有 16 女 54 3b CHC 否 利巴韦林 有 17 女 65 3b CHC 是 利巴韦林 有 18 女 58 3b CLC 是 利巴韦林 有 19 女 53 3b CLC、HCC 否 利巴韦林 有 20 女 59 3b CLC、HCC 否 利巴韦林 有 表 4 两组患者治疗前后生化指标比较
Table 4. Comparison of biochemical indicators before and after treatment in the two groups
项目 例数 基线 治疗4周 治疗12周 停药12周 χ2值 P值 TBil(μmol/L) CHC组 167 13.50(10.00~17.60) 15.70(11.60~19.90)1) 13.10(10.20~17.69)2) 15.31(14.82~16.03)3) 20.879 <0.001 CLC组 86 18.10(13.10~26.65) 18.54(14.53~25.78) 17.80(13.94~23.12) 15.39(14.44~16.34)1)2) 15.705 0.001 ALT(U/L) CHC组 167 51.00(32.00~93.00) 19.00(14.00~26.00)1) 18.00(14.00~23.00)1) 31.81(30.83~32.60)1)2)3) 263.638 <0.001 CLC组 86 71.50(41.75~113.50) 24.00(18.00~34.00)1) 24.00(19.00~34.11)1) 32.02(29.45~33.64)1)2)3) 123.074 <0.001 AST(U/L) CHC组 167 40.00(28.00~71.00) 24.00(20.00~29.00)1) 23.00(20.00~27.00)1) 37.22(35.76~37.90)2)3) 240.098 <0.001 CLC组 86 68.00(48.00~100.25) 30.00(24.74~40.25)1) 30.00(25.00~39.75)1) 37.13(35.54~38.15)1)2)3) 108.391 <0.001 注:1)与基线比较,P<0.05;2)与治疗4周比较,P<0.05;3)与治疗12周比较,P<0.05。
表 5 两组患者治疗前后肝脏LSM比较
Table 5. Comparison of liver LSM before and after treatment in the two groups
组别 例数 基线 治疗4周 治疗12周 停药12周 χ2值 P值 CLC组 167 14.65(11.95~20.35) 12.67(12.08~13.12)1) 12.97(11.68~14.07)2) 12.64(10.76~13.76)1) 42.235 <0.001 CHC组 86 13.68(6.90~14.76)2) 12.37(11.99~12.72)2) 11.90(10.53~12.98)2) 11.06(9.83~12.45) 37.606 <0.001 注:1)与基线比较,P<0.05;2)与停药12周比较,P<0.05。
表 6 两组患者治疗前后肾功能和血常规的指标比较
Table 6. Changes in renal function and blood routine before and after treatment in the two groups
项目 例数 基线 治疗4周 治疗12周 停药12周 χ2值 P值 PLT(×109/L) CHC组 167 191.00
(139.00~235.00)207.45
(161.85~258.00)1)206.00
(163.00~245.00)1)2)164.07
(162.38~166.33)1)2)3)56.674 <0.001 CLC组 86 123.00
(78.00~184.00)139.95
(102.00~190.86)144.50
(106.79~190.75)1)162.64
(141.00~165.56)10.924 0.012 WBC(×109/L) CHC组 167 5.46(4.41~6.55) 5.69(4.99~6.47) 5.63(4.84~6.44) 5.60(5.31~5.92) 5.538 0.136 CLC组 86 5.03(3.62~6.17) 5.54(4.24~6.85) 5.41(4.16~6.92) 5.55(5.23~5.97) 7.498 0.058 Cr(μmol/L) CHC组 167 60.00(50.00~71.00) 59.50(52.35~70.00) 62.00(53.00~69.40) 60.59(59.66~61.36) 1.897 0.594 CLC组 86 58.00(46.75~75.25) 57.93(49.75~69.25) 63.00(52.46~76.71) 60.78(58.99~61.81)3) 12.988 0.005 注:1)与基线比较,P<0.05;2)与治疗4周比较,P<0.05;3)与治疗12周比较,P<0.05。
表 7 服药及随访期间的不良事件
Table 7. Adverse events during medication and follow-up period
不良事件 总体(n=253) CHC组(n=167) CLC组(n=86) 死亡[例(%)] 0(0.00) 0(0.00) 0(0.00) 乏力[例(%)] 44(17.39) 37(22.16) 14(16.28) 恶心[例(%)] 6(2.37) 2(1.20) 4(4.65) 头晕[例(%)] 4(1.58) 3(1.80) 1(1.16) 皮疹[例(%)] 3(1.19) 1(0.60) 2(2.33) 皮肤瘙痒[例(%)] 1(0.40) 1(0.60) 0(0.00) 溶血性贫血[例(%)] 1(0.40) 0(0.00) 1(1.16) 头痛[例(%)] 1(0.40) 0(0.00) 1(1.16) -
[1] World Health Organization. Accountability for the global health secto strategies 2016-2021: Actions for impact[R]. Geneva: World Health Organization, 2021. [2] Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study[J]. Lancet Gastroenterol Hepatol, 2022, 7( 5): 396- 415. DOI: 10.1016/S2468-1253(21)00472-6. [3] RAY RB, RAY R. Hepatitis C virus manipulates humans as its favorite host for a long-term relationship[J]. Hepatology, 2019, 69( 2): 889- 900. DOI: 10.1002/hep.30214. [4] POORDAD F, SEDGHI S, POCKROS PJ, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir and dasabuvir with low-dose ribavirin in patients with chronic hepatitis C virus genotype 1a infection without cirrhosis[J]. J Viral Hepat, 2019, 26( 8): 1027- 1030. DOI: 10.1111/jvh.13109. [5] LI WC, LIANG J, AN JH, et al. Geographic distribution of HCV genotypes and efficacy of direct-acting antivirals in chronic HCV-infected patients in north and NorthEast China: A real-world multicenter study[J]. Can J Gastroenterol Hepatol, 2022, 2022: 7395506. DOI: 10.1155/2022/7395506. [6] HE N, FENG G, HAO S, et al. The impact of direct-acting antivirals on quality of life in patients with hepatitis C virus infection: A meta-analysis[J]. Ann Hepatol, 2022, 27( 4): 100705. DOI: 10.1016/j.aohep.2022.100705. [7] MOHD HANAFIAH K, GROEGER J, FLAXMAN AD, et al. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence[J]. Hepatology, 2013, 57( 4): 1333- 1342. DOI: 10.1002/hep.26141. [8] TOWNSHEND-BULSON L, ROIK E, BARBOUR Y, et al. The Alaska Native/American Indian experience of hepatitis C treatment with sofosbuvir-based direct-acting antivirals[J]. PLoS One, 2021, 16( 12): e0260970. DOI: 10.1371/journal.pone.0260970. [9] DAS D, PANDYA M. Recent advancement of direct-acting antiviral agents(DAAs) in hepatitis C therapy[J]. Mini Rev Med Chem, 2018, 18( 7): 584- 596. DOI: 10.2174/1389557517666170913111930. [10] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [11] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infections Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2022 version)[J]. Chin J Infect Dis, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 29- 46. DOI: 10.3760/cma.j.cn311365-20230217-00045. [12] GAO YH, KONG F, LI GM, et al. Coblopasvir and sofosbuvir for treatment of chronic hepatitis C virus infection in China: A single-arm, open-label, phase 3 trial[J]. Liver Int, 2020, 40( 11): 2685- 2693. DOI: 10.1111/liv.14633. [13] PAN CQ, PARK AJ, PARK JS. New perspectives in hepatocellular carcinoma surveillance after hepatitis C virus eradication[J]. Gastroenterol Rep(Oxf), 2024, 12: goae085. DOI: 10.1093/gastro/goae085. [14] WU N, RAO HY, YANG WB, et al. Impact of hepatitis C virus genotype 3 on liver disease progression in a Chinese national cohort[J]. Chin Med J(Engl), 2020, 133( 3): 253- 261. DOI: 10.1097/CM9.0000000000000629.DOI: 10.3969/j.issn.1001-5256.2023.03.009. [15] ZHANG W, ZHAI S, DU H, et al. Efficacy and safety of the 12-week sofosbuvir-coblopasvir regimen in treatment of chronic hepatitis C[J]. J Clin Hepatol, 2023, 39( 3): 539- 545. DOI: 10.3969/j.issn.1001-5256.2023.03.009.张伟, 翟嵩, 杜虹, 等. 12周索磷布韦联合可洛派韦治疗慢性丙型肝炎的效果和安全性分析[J]. 临床肝胆病杂志, 2023, 39( 3): 539- 545. DOI: 10.3969/j.issn.1001-5256.2023.03.009. [16] SHAO YL, XIA XS. Drug-resistance gene mutations and therapeutic drugs of the hepatitis C virus[J]. Chin J Virol, 2022, 38( 5): 1214- 1224. DOI: 10.13242/j.cnki.bingduxuebao.004124.邵榆岚, 夏雪山. 丙型肝炎病毒治疗药物与耐药基因突变[J]. 病毒学报, 2022, 38( 5): 1214- 1224. DOI: 10.13242/j.cnki.bingduxuebao.004124. [17] YE XT, XU S, ZHANG SG, et al. Efficacy and safety of sofosbuvir/velpatasvir in the treatment of patients with genotype 3 and 6 chronic hepatitis C[J]. J Wenzhou Med Univ, 2023, 53( 8): 662- 666. DOI: 10.3969/j.issn.2095-9400.2023.08.009.叶晓婷, 徐霜, 张盛果, 等. 索磷布韦维帕他韦治疗基因3型和6型慢性丙型肝炎患者的疗效和安全性[J]. 温州医科大学学报, 2023, 53( 8): 662- 666. DOI: 10.3969/j.issn.2095-9400.2023.08.009. [18] TANG Q. Clinical sofosbuvir-based therapies achieved satisfactory virological response in chinese with genotypes 3 and 6 infection: a real world experience[D]. Chongqing: Chongqing Medical University, 2021.唐巧. 索磷布韦为基础的治疗方案在HCV基因3型、6型患者中的有效性及安全性的真实世界研究[D]. 重庆: 重庆医科大学, 2021. [19] HLAING NT, MITRANI RA, AUNG ST, et al. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotypes 1-4 and 6 in Myanmar: Real-world experience[J]. J Viral Hepat, 2017, 24( 11): 927- 935. DOI: 10.1111/jvh.12721. [20] HUANG JN, JIANG JN, LIANG DD, et al. Epidemiological features and antiviral response of genotype 6 chronic hepatitis C[J]. J Clin Hepatol, 2022, 38( 4): 793- 797. DOI: 10.3969/j.issn.1001-5256.2022.04.011.黄锦妮, 江建宁, 梁丹丹, 等. 基因6型慢性丙型肝炎的流行病学特征及抗病毒疗效分析[J]. 临床肝胆病杂志, 2022, 38( 4): 793- 797. DOI: 10.3969/j.issn.1001-5256.2022.04.011. [21] BOKOCH MP, XU FY, GOVINDARAJU K, et al. Serum from patients with cirrhosis undergoing liver transplantation induces permeability in human pulmonary microvascular endothelial cells ex vivo[J]. Front Med(Lausanne), 2024, 11: 1412891. DOI: 10.3389/fmed.2024.1412891. [22] ÖZKAN A, STOLLEY DL, CRESSMAN ENK, et al. Vascularized hepatocellular carcinoma on a chip to control chemoresistance through cirrhosis, inflammation and metabolic activity[J]. Small Struct, 2023, 4( 9): 2200403. DOI: 10.1002/sstr.202200403. [23] BROCHADO-KITH Ó, MARTÍNEZ I, BERENGUER J, et al. HCV cure with direct-acting antivirals improves liver and immunological markers in HIV/HCV-coinfected patients[J]. Front Immunol, 2021, 12: 723196. DOI: 10.3389/fimmu.2021.723196. [24] AUMA AWN, SHIVE CL, KOSTADINOVA L, et al. Variable normalization of Naïve CD4+ lymphopenia and markers of monocyte and T cell activation over the course of direct-acting anti-viral treatment of chronic hepatitis C virus infection[J]. Viruses, 2021, 14( 1): 50. DOI: 10.3390/v14010050. [25] PIECHA F, GÄNßLER JM, OZGA AK, et al. Treatment and re-treatment results of HCV patients in the DAA era[J]. PLoS One, 2020, 15( 5): e0232773. DOI: 10.1371/journal.pone.0232773. [26] ZHU MY, YU P, GE GH, et al. Efficacy and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous direct-acting antiviral agent failure[J]. J Clin Hepatol, 2024, 40( 11): 2201- 2204. DOI: 10.12449/JCH241112.朱梦莹, 俞萍, 葛国洪, 等. 索磷布韦/维帕他韦/伏西瑞韦治疗既往直接抗病毒药物治疗失败的慢性丙型肝炎患者的有效性和安全性[J]. 临床肝胆病杂志, 2024, 40( 11): 2201- 2204. DOI: 10.12449/JCH241112. [27] CHEN SS, YAN R, ZHOU K, et al. Efficacy of SOF/VEL/VOX retreatment in DAAs-failed chronic hepatitis C patients with different genotypes[J]. Chin J Infect Dis, 2023, 16( 5): 372- 376. DOI: 10.3760/cma.j.issn.1674-2397.2023.05.006.陈闪闪, 严蓉, 周克, 等. 索磷布韦/维帕他韦/伏西瑞韦再治疗DAAs治疗失败的不同基因型慢性丙型肝炎患者的疗效分析[J]. 中华临床感染病杂志, 2023, 16( 5): 372- 376. DOI: 10.3760/cma.j.issn.1674-2397.2023.05.006. [28] CHEN CY, HUANG CF, CHENG PN, et al. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan[J]. Liver Int, 2021, 41( 6): 1265- 1277. DOI: 10.1111/liv.14849. [29] OGAWA E, TOYODA H, IIO E, et al. Hepatitis C virus cure rates are reduced in patients with active but not inactive hepatocellular carcinoma: A practice implication[J]. Clin Infect Dis, 2020, 71( 11): 2840- 2848. DOI: 10.1093/cid/ciz1160. [30] PATEL SV, JAYAWEERA DT, ALTHOFF KN, et al. Real-world efficacy of direct acting antiviral therapies in patients with HIV/HCV[J]. PLoS One, 2020, 15( 2): e0228847. DOI: 10.1371/journal.pone.0228847. [31] LIU L, CHANG LX, CHEN ZY, et al. Efficacy and safety of sofosbuvir/velpatasvir alone or in combination with ribavirin in treatment of patients with genotype 3B HCV/HIV infection[J]. J Clin Hepatol, 2024, 40( 2): 271- 277. DOI: 10.12449/JCH240209.刘立, 常丽仙, 陈智勇, 等. 索磷布韦/维帕他韦单用或联合利巴韦林治疗3B型HCV/HIV感染者的效果及安全性[J]. 临床肝胆病杂志, 2024, 40( 2): 271- 277. DOI: 10.12449/JCH240209. -



 PDF下载 ( 1348 KB)
PDF下载 ( 1348 KB)


 下载:
下载: