补体应答基因32在小鼠部分肝切除后肝再生过程中的表达及意义
DOI: 10.3969/j.issn.1001-5256.2023.10.018
伦理学声明:本研究方案于2021年12月5日经由湖北医药学院实验动物伦理委员会审批,批号:2021-实071号,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:孔德营、唐俊明负责课题设计;李兴元、杨艳芳、陈琰、胡文慧参与动物模型的构建;李兴元、杨艳芳负责做实验、整理数据及撰写论文;赵小英负责对论文进行修改;李兴元和杨艳芳对本文贡献等同,同为第一作者。
Expression and significance of response gene to complement 32 in liver regeneration after partial hepatectomy in mice
-
摘要:
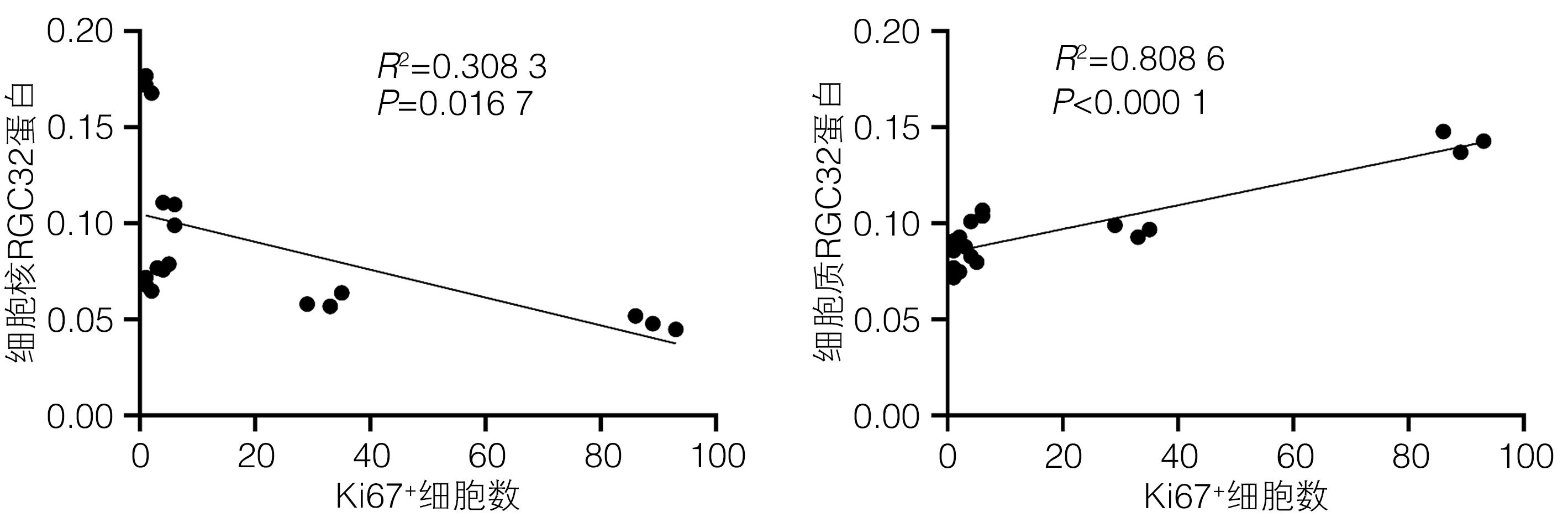
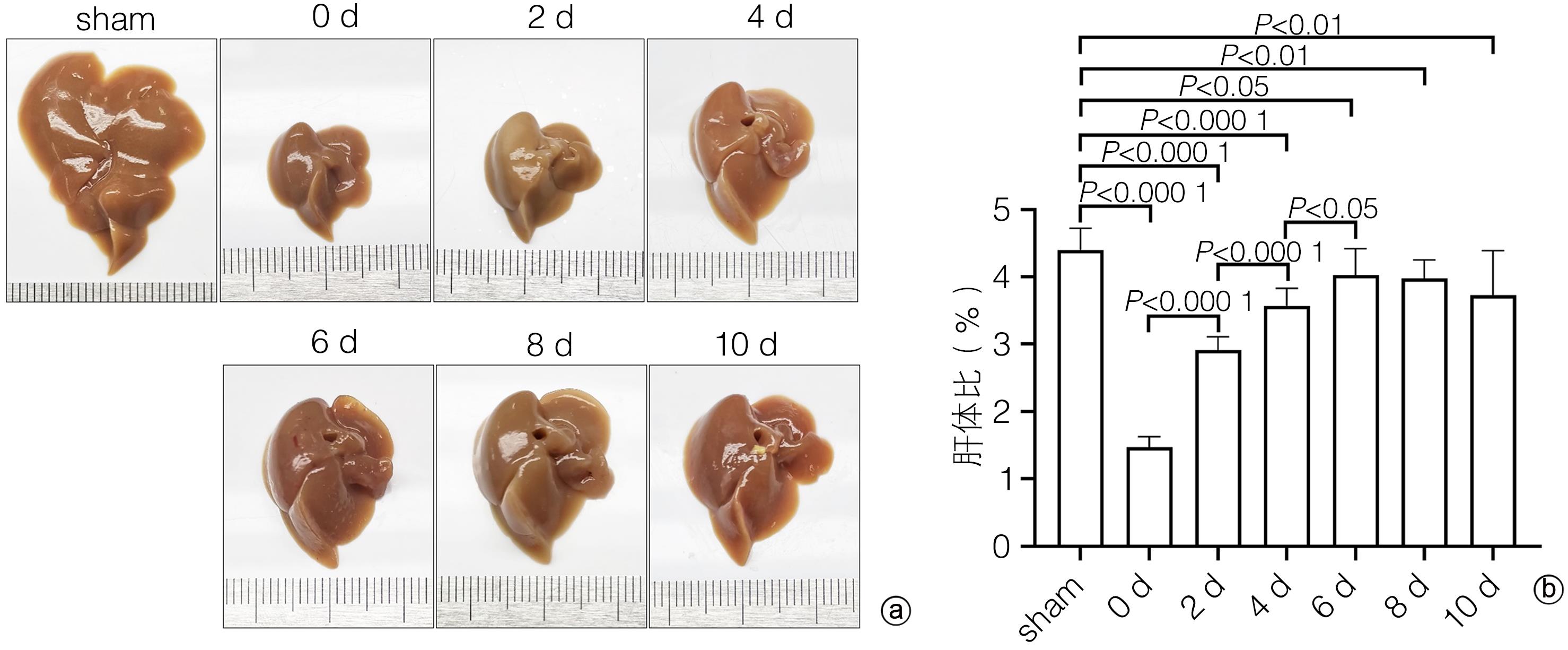
目的 探讨补体应答基因32(RGC32)在部分肝切除(PH)术后肝再生过程中的表达及作用。 方法 42只10周龄雄性C57BL/6小鼠,随机分为对照组[切除小鼠完整的肝脏称重、拍照作为正常对照(sham组),进一步切除肝左叶和肝中叶后称重、拍照作为手术对照(0 d组),sham组和0 d组共用一组小鼠]、术后1天组(1 d)、术后2天组(2 d)、术后4天组(4 d)、术后6天组(6 d)、术后8天组(8 d)和术后10天组(10 d),每组6只;PH术建模成功后分别于术后1、2、4、6、8、10天处死小鼠,收集小鼠肝脏,检测肝脏大小变化。HE和油红O染色评估肝组织形态学变化,血清ALT、AST检测评价肝功能变化,免疫组化染色检测增殖细胞核抗原(PCNA)和Ki67表达并分析肝再生过程中细胞增殖变化,实时荧光定量PCR和免疫组化染色技术检测肝再生过程中RGC32的表达及其亚细胞分布。EdU细胞增殖实验分析L02细胞过表达或者敲除RGC32对肝细胞增殖的影响。计量资料多组间比较采用方差分析,进一步两两比较采用LSD-t检验;两组间比较采用成组t检验。相关性分析采用Pearson相关分析法。 结果 PH术后肝脏逐渐增大,第0~6天为肝体比(肝脏质量/体质量)上升的高峰期,不同时间点比较差异均有统计学意义(P值均<0.05);第6~10天肝脏大小变化不明显。PH术后肝脏脂滴显著增多,随着肝再生,脂滴减少,且呈现门静脉区和中央静脉区差异化(P值均<0.05)。与sham组比较,PH术后1天,血清ALT、AST水平明显升高(P值均<0.05),随后分别于术后第6天和术后第2天恢复至sham组水平(P值均>0.05)。免疫组化染色结果显示,PH术后PCNA和Ki67阳性肝实质细胞数迅速增多,第2天数目最多,分别为86±5和89±5,随后逐渐减少;而PCNA和Ki67阳性非实质细胞数逐渐增多,第6天才达到高峰,分别为34±5和25±3,随后逐渐减少。PH术后总RGC32表达在第2天升到最高,随后逐渐降低,细胞质RGC32表达变化趋势与之一致;而细胞核RGC32的表达在PH术后第2天降到最低,随后逐渐升高。相关性分析显示,细胞核RGC32的表达与肝实质细胞的增殖呈负相关(R2=0.308 3,P=0.016 7),细胞质RGC32的表达与肝实质细胞的增殖呈正相关(R2=0.808 6,P<0.000 1)。细胞实验显示,与对照组相比,RGC32过表达EdU阳性率减少15.6%(P<0.01),敲低RGC32表达EdU阳性率增加19.2%(P<0.01)。 结论 肝实质细胞和非实质细胞增殖不同步,共同参与了肝脏再生。肝切除术后肝再生过程中,RGC32表达存在核质分布差异,核RGC32对肝细胞增殖具有抑制作用。 -
关键词:
- 补体应答基因32 /
- 肝切除术 /
- 肝再生 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the expression and role of response gene to complement 32 (RGC32) in liver regeneration after partial hepatectomy (PH). Methods A total of 42 male C57BL/6 mice, aged 10 weeks, were randomly divided into control group, postoperative day 1 group (1-d group), postoperative day 2 group (2-d group), postoperative day 4 group (4-d group), postoperative day 6 group (6-d group), postoperative day 8 group (8-d group), and postoperative day 10 group (10-d group), with 6 mice in each group. In the control group, the complete liver of the mice was resected for weighing and photography as the normal control group (sham group); further, the left and middle lobes of the liver were resected for weighing and photography as the surgical control group (0-day group); the sham group and the 0-day group shared the same group of mice. After successful modeling by PH, the mice were sacrificed on days 1, 2, 4, 6, 8, and 10 after surgery, and the liver was collected to measure the change in size. HE staining and oil red O staining were used to evaluate liver histomorphological changes; serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured to evaluate the changes in liver function; immunohistochemical staining was used to measure the expression of proliferating cell nuclear antigen (PCNA) and Ki67 and analyze the change in cell proliferation during liver regeneration; quantitatie real-time PCR and immunohistochemical staining were uused to measure the expression and subcellular distribution of RGC32 during liver regeneration; EdU cell proliferation assay was used to analyze the effect of RGC32 overexpression or knocknout on hepatocyte proliferation in L02 cells. For continuous data, comparison between multiple groups was made by analysis of variance, and further pairwise comparisons were conducted using the LSD-t test. The independent samples t-test was used for comparison of continuous data between two groups. A Pearson correlation analysis was performed. Results The liver gradually enlarged after PH, and the liver/body weight ratio rose to the peak from days 0 to 6, with significant differences between different time points (all P<0.05), while there was no significant change in liver size from days 6 to 10. The number of liver lipid droplets significantly increased after PH surgery and gradually decreased with liver regeneration, with a significant difference between the portal vein region and the central vein region (all P<0.05). Compared with the sham group, the 1d group had significant increases in the serum levels of ALT and AST (all P<0.05), which gradually returned to the levels of the sham group on day 6 and day 2 after surgery, respectively (P>0.05). Immunohistochemical staining showed that there were rapid increases in the numbers of PCNA- and Ki67-positive liver parenchymal cells after PH surgery, with the highest numbers of 86±5 and 89±5, respectively, on day 2, which then gradually decreased; however, there were gradual increases in the numbers of PCNA- and Ki67-positive nonparenchymal cells, with the peak numbers of 34±5 and 25±3, respectively, on day 6, which then gradually decreased. The total expression of RGC32 increased to the highest level on day 2 after PH surgery and then gradually decreased, and the changing trend of RGC32 expression in cytoplasm was consistent with that of total RGC32 expression; however, the expression of RGC32 in nucleus decreased to the lowest level on day 2 after PH surgery and then increased gradually. The correlation analysis showed that the expression of RGC32 in nucleus was negatively correlated with the proliferation of liver parenchymal cells (R2=0.308 3, P=0.016 7), and the expression of RGC32 in cytoplasm was positively correlated with the proliferation of liver parenchymal cells (R2=0.808 6, P<0.000 1). Cell experiments showed that compared with the control group, the EdU-positive rate was reduced by 15.6% after RGC32 overexpression (P<0.01) and was increased by 19.2% after RGC32 knockdown (P<0.01). Conclusion Liver parenchymal cells and nonparenchymal cells show asynchronous proliferation and participate in liver regeneration together. During liver regeneration after hepatectomy, there are differences in the expression of RGC32 between nucleus and cytoplasm, and RGC32 in nucleus may inhibit hepatocyte proliferation. -
Key words:
- RGC32 /
- Hepatectomy /
- Liver Regeneration /
- Mice, Inbred C57BL
-
-
[1] MICHALOPOULOS GK, BHUSHAN B. Liver regeneration: biological and pathological mechanisms and implications[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 1): 40- 55. DOI: 10.1038/s41575-020-0342-4. [2] LIN S, NASCIMENTO EM, GAJERA CR, et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury[J]. Nature, 2018, 556( 7700): 244- 248. DOI: 10.1038/s41586-018-0004-7. [3] CHEN HX, HUANG SJ, CHEN JY, et al. Role of complement in liver injury and regeneration[J]. Central South Pharm, 2021, 19( 10): 2123- 2127. DOI: 10.7539/j.issn.1672-2981.2021.10.023.陈海霞, 黄少杰, 陈锦仪, 等. 补体在肝脏损伤与再生中的作用[J]. 中南药学, 2021, 19( 10): 2123- 2127. DOI: 10.7539/j.issn.1672-2981.2021.10.023. [4] WANG Q, QU X. New insights into the roles of RGC32[J]. Cell Mol Immunol, 2018, 15( 8): 803- 804. DOI: 10.1038/cmi.2017.154. [5] LI JX, LI MY. Research progress in animal models of regeneration[J]. Med Recapitulate, 2021, 27( 23): 4629- 4633. DOI: 10.3969/j.issn.1006-2084.2021.23.011.李嘉兴, 李明意. 肝脏再生动物模型研究进展[J]. 医学综述, 2021, 27( 23): 4629- 4633. DOI: 10.3969/j.issn.1006-2084.2021.23.011. [6] NEVZOROVA YA, TOLBA R, TRAUTWEIN C, et al. Partial hepatectomy in mice[J]. Lab Anim, 2015, 49( Suppl 1): 81- 88. DOI: 10.1177/0023677215572000. [7] LÓPEZ-LUQUE J, FABREGAT I. Revisiting the liver: from development to regeneration-what we ought to know![J]. Int J Dev Biol, 2018, 62( 6- 7- 8): 441- 451. DOI: 10.1387/ijdb.170264JL. [8] van HAELE M, SNOECK J, ROSKAMS T. Human liver regeneration: an etiology dependent process[J]. Int J Mol Sci, 2019, 20( 9): 2332. DOI: 10.3390/ijms20092332. [9] YAGI S, HIRATA M, MIYACHI Y, et al. Liver regeneration after hepatectomy and partial liver transplantation[J]. Int J Mol Sci, 2020, 21( 21): 8414. DOI: 10.3390/ijms21218414. [10] YU ZY, LIN X, HAN Y, et al. Role of liver sinusoidal endothelial cells in liver regeneration and the development of liver fibrosis[J]. J Clin Hepatol, 2019, 35( 9): 2072- 2074. DOI: 10.3969/j.issn.1001-5256.2019.09.041.于子越, 蔺鑫, 韩英, 等. 肝血窦内皮细胞在肝再生和肝纤维化发生中的作用[J]. 临床肝胆病杂志, 2019, 35( 9): 2072- 2074. DOI: 10.3969/j.issn.1001-5256.2019.09.041. [11] LI N, LIU C, MA G, et al. Asparaginyl endopeptidase may promote liver sinusoidal endothelial cell angiogenesis via PI3K/Akt pathway[J]. Rev Esp Enferm Dig, 2019, 111( 3): 214- 222. DOI: 10.17235/reed.2018.5709/2018. [12] VLAICU SI, TATOMIR A, ANSELMO F, et al. RGC32 and diseases: the first 20 years[J]. Immunol Res, 2019, 67( 2- 3): 267- 279. DOI: 10.1007/s12026-019-09080-0. [13] YU L, YU YH. Research of RGC32 in tumor[J]. Mil Med J Southeast China, 2020, 22( 1): 71- 75. DOI: 10.3969/j.issn.1672-271X.2020.01.016.禹乐, 余英豪. RGC32在肿瘤中的研究进展[J]. 东南国防医药, 2020, 22( 1): 71- 75. DOI: 10.3969/j.issn.1672-271X.2020.01.016. [14] VLAICU SI, TATOMIR A, BOODHOO D, et al. RGC32 is expressed in the human atherosclerotic arterial wall: Role in C5b-9-induced cell proliferation and migration[J]. Exp Mol Pathol, 2016, 101( 2): 221- 230. DOI: 10.1016/j.yexmp.2016.09.004. [15] CUI XB, GUO X, CHEN SY. Response gene to complement 32 deficiency causes impaired placental angiogenesis in mice[J]. Cardiovasc Res, 2013, 99( 4): 632- 639. DOI: 10.1093/cvr/cvt121. [16] CUI XB, LUAN JN, DONG K, et al. RGC32(response gene to complement 32) deficiency protects endothelial cells from inflammation and attenuates atherosclerosis[J]. Arterioscler Thromb Vasc Biol, 2018, 38( 4): e36-e47. DOI: 10.1161/ATVBAHA.117.310656. [17] HU C, WU Z, LI L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells[J]. Int J Biol Sci, 2020, 16( 5): 893- 903. DOI: 10.7150/ijbs.39725. [18] ZHENG J, LI JT, JIN J, et al. Characteristics of incision bacterial infection and changes of immune cytokines in patients with hepatocellular carcinoma after hepatectomy[J]. Chin J Microecol, 2022, 34( 7): 819- 823, 845. DOI: 10.13381/j.cnki.cjm.202207014.郑杰, 李江涛, 金晶, 等. 肝癌患者肝切除术后切口细菌感染特征及免疫细胞因子水平变化[J]. 中国微生态学杂志, 2022, 34( 7): 819- 823, 845. DOI: 10.13381/j.cnki.cjm.202207014. [19] SAIGUSA K, IMOTO I, TANIKAWA C, et al. RGC32, a novel p53-inducible gene, is located on centrosomes during mitosis and results in G2/M arrest[J]. Oncogene, 2007, 26( 8): 1110- 1121. DOI: 10.1038/sj.onc.1210148. -



 PDF下载 ( 3088 KB)
PDF下载 ( 3088 KB)

 下载:
下载: