累积血浆致动脉硬化指数预测中青年人群非酒精性脂肪性肝病发病风险的队列研究
DOI: 10.12449/JCH251113
A cohort study on cumulative atherogenic index of plasma for predicting the risk of developing new-onset non-alcoholic fatty liver disease in a population of young and middle-aged individuals
-
摘要:
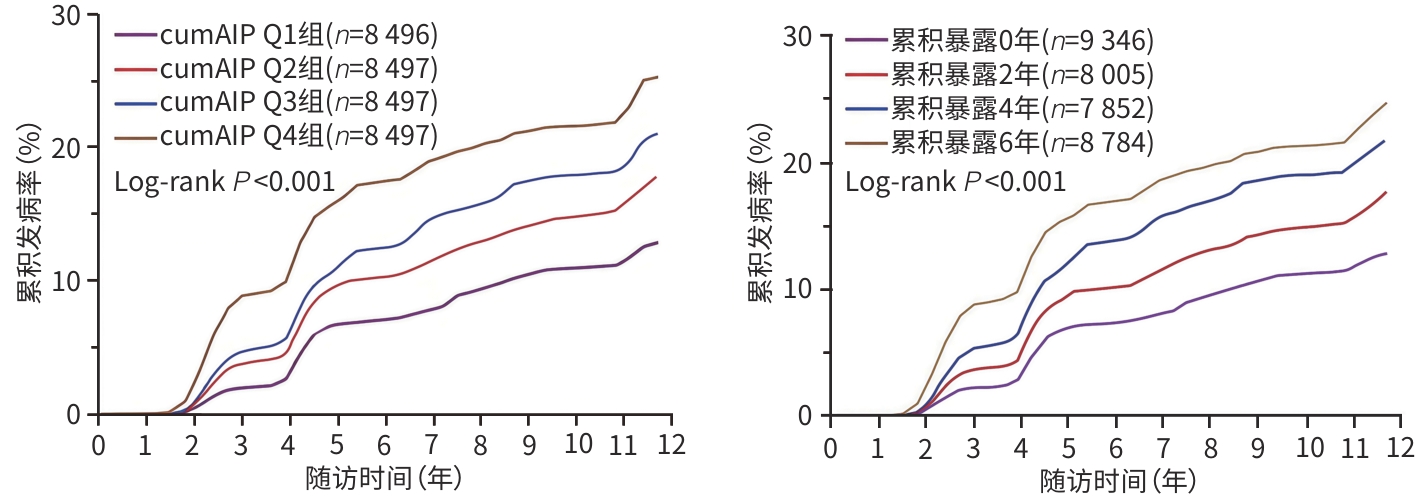
目的 探讨累积血浆致动脉硬化指数(cumAIP)与年龄<60岁人群新发非酒精性脂肪性肝病(NAFLD)风险的关联。 方法 采用前瞻性队列研究方法,选取2006年6月—2010年10月在开滦总医院及其下属10家医院完成健康体检的开滦研究队列中青年人群(年龄18~<60岁)为研究对象,根据纳入与排除标准筛选后,33 987例进入观察队列。根据计算的cumAIP值四分位数分为4组(Q1、Q2、Q3、Q4组),用Kaplan-Meier法计算4组NAFLD的累积发病率,并使用Log-rank检验比较组间差异;采用多因素Cox回归分析获得4组中新发NAFLD风险的风险比(HR)和95%可信区间(CI)。正态分布的计量资料多组间比较采用单因素方差分析,非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验;分类变量组间比较采用χ2检验。 结果 平均随访(10.89±2.54)年,共新发NAFLD 6 011例,cumAIP Q1~Q4组分别发生995例、1 366例、1 661例和1 989例,发病密度依次为11.37/1 000人年、16.02/1 000人年、19.97/1 000人年和24.91/1 000人年。Log-rank检验显示,各分组之间累积发病率差异有统计学意义(P<0.001)。以是否发生NAFLD为因变量,不同cumAIP暴露水平四分位数分组为自变量,以cumAIP Q1组为参照,多因素Cox回归模型显示:Q2、Q3、Q4组新发NAFLD的HR及95%CI分别为1.30(1.20~1.41)、1.52(1.41~1.65)和1.79(1.64~1.95),P趋势<0.001。以是否新发NAFLD为因变量,AIP累积暴露0、2、4和6年为自变量,以AIP累积暴露0年为参照,Cox回归分析显示:AIP累计暴露2、4、6年的HR及95%CI分别为1.24(1.15~1.35)、1.51(1.40~1.64)和1.70(1.56~1.84),P趋势<0.001。在排除2年内新发NAFLD,随访期间发生ASCVD事件患者,服用降压药、降糖药、降脂药的研究对象后,分别进行敏感性分析,结果与主要分析结果相似;考虑到全因死亡与结局事件存在竞争关系,进行死亡竞争风险分析,结果显示,风险分析结果与主要分析结果相似。 结论 cumAIP高水平暴露增加中青年人群新发NAFLD风险。 Abstract:Objective To investigate the association between cumulative atherogenic index of plasma (cumAIP) and the risk of new-onset nonalcoholic fatty liver disease (NAFLD) in young and middle-aged individuals. Methods A prospective cohort study was conducted among the young and middle-aged individuals (aged 18 to <60 years) in the Kailuan study cohort who underwent physical examination in Kailuan General Hospital and its 10 affiliated hospitals in June 2006 to October 2010, and after screening based on the inclusion and exclusion criteria, 33 987 individuals were included in the observation cohort. The individuals were divided into Q1, Q2, Q3, and Q4 groups based on the quantiles of cumAIP. The Kaplan-Meier method was used to calculate the cumulative incidence rate of new-onset NAFLD in the four groups, while the log-rank test was used for comparison between groups. A multivariate Cox regression analysis was used to obtain the hazard ratio (HR) and 95% confidence interval (CI) of the risk of new-onset NAFLD in the four groups. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups; the chi-square test was used for comparison of categorical variables between groups. Results The mean follow-up was 10.89±2.54 years, and there were 6 011 cases of new-onset NAFLD, including 995 cases in the Q1 group, 1 366 in the Q2 group, 1661 in the Q3 group, and 1 989 in the Q4 group, with an incidence density of 11.37, 16.02, 19.97, and 24.91 per thousand person-years. The log-rank test showed that there was a significant difference in cumulative incidence rate between the four groups (P<0.001). With the presence or absence of NAFLD as the dependent variable and the quantiles of different exposure levels to cumAIP as the independent variable, the multivariate Cox regression model analysis showed that compared with the Q1 group, the Q2, Q3, and Q4 groups had an HR of 1.30 (95%CI: 1.20 — 1.41), 1.52 (95%CI: 1.41 — 1.65), and 1.79 (95%CI: 1.64 — 1.95), respectively, for new-onset NAFLD, with a Ptrend value of <0.001. With the presence or absence of new-onset NAFLD as the dependent variable and the cumulative exposure to AIP for 0, 2, 4, and 6 years as the independent variable, the Cox regression analysis showed that compared with cumulative exposure to AIP for 0 years, cumulative exposure to AIP for 2, 4, and 6 years had an HR of 1.24 (95%CI: 1.15 — 1.35), 1.51 (95%CI: 1.40 — 1.64), and 1.70 (95%CI: 1.56 — 1.84), respectively, with a Ptrend value of <0.001. A sensitivity analysis was performed after exclusion of the individuals with new-onset NAFLD within 2 years, the individuals who experienced atherosclerotic cardiovascular disease events during follow-up, and the individuals taking antihypertensive, hypoglycemic, and lipid-lowering drugs, and the results were similar to those of the main analysis. Considering the competitive relationship between all-cause death and outcome events, a competing risk analysis of death was performed, which showed that the results of risk analysis were similar to those of the main analysis. Conclusion A high level of cumAIP exposure can increase the risk of new-onset NAFLD in young and middle-aged individuals. -
表 1 不同cumAIP水平分组研究对象一般情况
Table 1. The basic conditions of different cumAIP level group
项目 总计
(n=33 987)cumAIP四分位数分组 统计值 P值 Q1组(n=8 496) Q2组(n=8 497) Q3组(n=8 497) Q4组(n=8 497) cumAIP -0.18±0.58 -0.88±0.24 -0.38±0.11 -0.04±0.10 0.57±0.38 F=478.05 <0.001 年龄(岁) 49.33±9.32 48.94±9.46 49.31±9.48 49.30±9.38 49.74±8.93 F=3 213.58 <0.001 BMI(kg/m2) 24.42±3.03 23.25±2.98 24.19±3.00 24.73±2.85 25.49±2.83 F=760.19 <0.001 腰围(cm) 85.93±9.74 82.40±9.69 85.12±9.73 86.75±9.31 89.44±8.83 F=270.63 <0.001 SBP(mmHg) 126.80±18.00 122.96±17.90 126.04±17.80 127.69±17.61 130.52±17.88 F=31.20 <0.001 DBP(mmHg) 82.98±10.62 80.23±10.44 82.35±10.52 83.88±10.35 85.48±10.44 F=5.90 <0.001 ALT(U/L) 19.12±16.77 16.52±18.06 18.04±16.04 19.89±13.71 22.03±18.33 F=67.56 <0.001 FBG(mmol/L) 5.49±1.70 5.27±1.46 5.42±1.55 5.53±1.99 5.73±1.73 F=5.52 <0.001 TG(mmol/L) 1.20(0.87~1.74) 0.75(0.58~0.97) 1.08(0.86~1.36) 1.35(1.10~1.72) 2.13(1.51~3.08) H=431.82 <0.001 TC(mmol/L) 4.93±1.16 4.80±1.02 4.87±1.18 4.93±1.33 5.11±1.06 F=14.39 <0.001 HDL-C(mmol/L) 1.58±0.48 1.85±0.54 1.61±0.44 1.48±0.38 1.37±0.41 F=178.98 <0.001 LDL-C(mmol/L) 2.56±0.96 2.36±0.77 2.59±0.77 2.65±0.79 2.64±1.35 F=6.66 <0.001 hs-CRP(mg/L) 0.92(0.40~2.10) 0.80(0.40~1.90) 0.93(0.49~2.00) 0.86(0.30~2.00) 1.10(0.50~2.47) H=145.79 <0.001 RHR(次/min) 73.00±10.14 71.99±9.95 72.90±10.10 73.20±10.15 73.90±10.26 F=57.84 <0.001 AIP2006 -0.19±0.66 -0.80±0.42 -0.38±0.40 -0.08±0.42 0.49±0.59 F=312.44 <0.001 AIP2008 -0.18±0.68 -0.92±0.35 -0.39±0.28 -0.02±0.27 0.62±0.55 F=283.32 <0.001 AIP2010 -0.18±0.71 -0.86±0.46 -0.36±0.42 -0.03±0.42 0.54±0.64 F=536.21 <0.001 男性[例(%)] 24 742(72.80) 5 196(61.16) 5 910(69.55) 6 512(76.64) 7 124(83.84) χ2=28.49 <0.001 高中及以上教育程度
[例(%)]10 239(30.13) 2 549(30.00) 2 531(29.79) 2 662(31.33) 2 497(29.39) χ2=188.52 0.073 人均月收入≥1 000元
[例(%)]17 787(52.33) 4 441(52.27) 4 473(52.64) 4 280(50.37) 4 593(54.05) χ2=169.83 <0.001 体育锻炼[例(%)] 22 229(65.40) 5 360(63.09) 5 576(65.62) 5 713(67.24) 5 580(65.67) χ2=14.51 <0.001 吸烟史[例(%)] 11 468(33.74) 2 320(27.31) 2 759(32.47) 2 838(33.40) 3 551(41.79) χ2=36.80 <0.001 糖尿病史[例(%)] 2 308(6.79) 339(3.99) 474(5.58) 584(6.87) 911(10.72) χ2=29.55 <0.001 高血压病史[例(%)] 12 347(36.33) 2 324(27.35) 2 843(33.46) 3 258(38.34) 3 922(46.16) χ2=46.51 <0.001 血脂异常[例(%)] 17 656(51.95) 2 906(34.20) 3 562(41.92) 4 338(51.05) 6 850(80.62) χ2=47.55 <0.001 服降糖药[例(%)] 1 205(3.55) 201(2.37) 240(2.82) 287(3.38) 477(5.61) χ2=17.95 <0.001 服降压药[例(%)] 4 461(13.13) 673(7.92) 963(11.33) 1 191(14.02) 1 634(19.23) χ2=48.24 <0.001 服降脂药[例(%)] 2 164(6.37) 331(3.90) 468(5.51) 538(6.33) 827(9.73) χ2=12.09 <0.001 表 2 AIP累积暴露对NAFLD影响的多因素Cox回归分析
Table 2. Multivariate Cox regression analysis of the effects of cumAIP exposure on NAFLD
参数 发病人数
[例(%)]随访时间
(人年)发病密度
(/1 000人年)HR(95%CI) 模型1 模型2 模型3 cumAIP Q1组(n=8 496) 995(11.71) 87 500 11.37(10.69~12.10) 1.00 1.00 1.00 Q2组(n=8 497) 1 366(16.08) 85 245 16.02(15.20~16.90) 1.44(1.33~1.56) 1.29(1.19~1.41) 1.30(1.20~1.41) Q3组(n=8 497) 1 661(19.55) 83 181 19.97(19.03~20.95) 1.80(1.66~1.95) 1.52(1.41~1.65) 1.52(1.41~1.65) Q4组(n=8 497) 1 989(23.41) 79 859 24.91(23.84~26.03) 2.39(2.20~2.58) 1.81(1.67~1.97) 1.79(1.64~1.95) P趋势 <0.001 <0.001 <0.001 AIP累积暴露时间 0年(n=9 346) 1 107(11.84) 96 162 11.51(10.85~12.21) 1.00 1.00 1.00 2年(n=8 005) 1 290(16.11) 80 241 16.08(15.22~16.98) 1.39(1.28~1.51) 1.25(1.15~1.36) 1.24(1.15~1.35) 4年(n=7 852) 1 599(20.36) 76 502 20.91(19.90~21.95) 1.81(1.68~1.96) 1.53(1.41~1.66) 1.51(1.40~1.64) 6年(n=8 784) 2 015(22.94) 82 880 24.31(23.27~25.40) 2.25(2.09~2.43) 1.73(1.60~1.87) 1.70(1.56~1.84) P趋势 <0.001 <0.001 <0.001 注:模型1校正基线年龄和性别;模型2在模型1的基础上校正基线BMI、腰围、SBP、ALT、TC、FBG、RHR、吸烟史;模型3在模型2的基础上校正教育水平、体育锻炼、收入水平、病史(高血压、糖尿病、血脂异常)、服药史(降压药、降糖药、降脂药)。
表 3 cumAIP暴露水平及累计暴露时间对新发NAFLD风险影响的敏感性分析
Table 3. Sensitivity analysis of cumAIP exposure level and cumulative exposure time on the risk of new-onset NAFLD
项目 发病人数/总人数 HR(95%CI)1) 项目 发病人数/总人数 HR(95%CI)1) cumAIP AIP累积暴露时间 敏感性分析1(n=33 408) 敏感性分析1(n=33 408) Q1组 935/8 436 1.00 0年 1 040/9 279 1.00 Q2组 1 274/8 405 1.31(1.21~1.43) 2年 1 197/7 912 1.25(1.15~1.36) Q3组 1 513/8 349 1.53(1.41~1.66) 4年 1 460/7 713 1.53(1.40~1.66) Q4组 1 710/8 218 1.75(1.60~1.92) 6年 1 735/8 504 1.66(1.52~1.81) P趋势 <0.001 P趋势 <0.001 敏感性分析2(n=31 604) 敏感性分析2(n=31 604) Q1组 954/8 060 1.00 0年 1 066/8 872 1.00 Q2组 1 298/7 976 1.30(1.19~1.41) 2年 1 221/7 513 1.24(1.14~1.32) Q3组 1 567/7 880 1.53(1.41~1.67) 4年 1 491/7 223 1.51(1.39~1.64) Q4组 1 843/7 688 1.81(1.66~1.98) 6年 1 884/7 996 1.73(1.59~1.88) P趋势 <0.001 P趋势 <0.001 敏感性分析3(n=30 553) 敏感性分析3(n=30 553) Q1组 957/8 002 1.00 0年 1 055/8 753 1.00 Q2组 1 284/7 756 1.29(1.18~1.40) 2年 1 215/7 353 1.24(1.14~1.35) Q3组 1 556/7 618 1.52(1.40~1.65) 4年 1 491/6 988 1.52(1.40~1.65) Q4组 1 779/7 177 1.79(1.64~1.95) 6年 1 815/7 459 1.72(1.58~1.87) P趋势 <0.001 P趋势 <0.001 死亡竞争风险(n=33 987) 死亡竞争风险(n=33 987) Q1组 995/8 496 1.00 0年 1 107/9 346 1.00 Q2组 1 366/8 497 1.30(1.20~1.41) 2年 1 290/8 005 1.24(1.15~1.35) Q3组 1 661/8 497 1.53(1.40~1.66) 4年 1 599/7 852 1.51(1.40~1.64) Q4组 1 989/8 497 1.79(1.64~1.95) 6年 2 015/8 784 1.70(1.56~1.84) P趋势 <0.001 P趋势 <0.001 注:1)模型校正了年龄、性别、BMI、腰围、SBP、ALT、TC、FBG、RHR、吸烟史、教育水平、体育锻炼、收入水平、病史(高血压、糖尿病、血脂异常)、服药史(降压药、降糖药、降脂药)。敏感性分析1:排除2年内新发NAFLD患者;敏感性分析2:排除随访期间发生ASCVD事件者;敏感性分析3:排除服用降压药、降糖药、降脂药者。
表 4 采用C指数、NRI、IDI比较BMI、AIP2006与cumAIP对NAFLD发生风险的预测价值
Table 4. Comparison of the predictive value for NAFLD risk between BMI, AIP2006, and cumAIP using the C-index, NRI, and IDI
项目 C指数(95%CI) NRI(95%CI) P值 IDI(95%CI) P值 原模型 0.722 5(0.716 9~0.728 1) 原模型+BMI 0.732 7(0.727 2~0.738 3) 0.235 3(0.207 6~0.263 1) <0.001 0.005 9(0.004 9~0.006 9) <0.001 原模型+AIP2006 0.727 4(0.721 8~0.733 0) 0.202 0(0.174 2~0.229 7) <0.001 0.004 5(0.003 6~0.005 3) <0.001 原模型+cumAIP 0.728 4(0.722 8~0.734 0) 0.211 6(0.183 8~0.239 4) <0.001 0.004 8(0.003 9~0.005 7) <0.001 注:原模型校正了基线年龄、性别、腰围、SBP、ALT、TC、FBG、RHR、吸烟史、教育水平、体育锻炼、收入水平、病史(高血压、糖尿病、血脂异常)、服药史(降压药、降糖药、降脂药)。
-
[1] RIAZI K, AZHARI H, CHARETTE JH, et al. The prevalence and incidence of NAFLD worldwide: A systematic review and Meta-analysis[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 851- 861. DOI: 10.1016/S2468-1253(22)00165-0. [2] YIP TC, LEE HW, CHAN WK, et al. Asian perspective on NAFLD-associated HCC[J]. J Hepatol, 2022, 76( 3): 726- 734. DOI: 10.1016/j.jhep.2021.09.024. [3] ZHOU ZF, LI Y, HUANG SQ, et al. Study on the relationship between serum uric acid and non-alcoholic fatty liver disease in physical examination population aged 18 to 60 years in Luzhou city[J]. Mod Prev Med, 2023, 50( 9): 1722- 1728. DOI: 10.20043/j.cnki.MPM.202301105.周仲芳, 李洋, 黄素琼, 等. 泸州市18~60岁体检人群血尿酸水平与非酒精性脂肪肝关系研究[J]. 现代预防医学, 2023, 50( 9): 1722- 1728. DOI: 10.20043/j.cnki.MPM.202301105. [4] ZHANG YX, WANG Y, YOU CL, et al. Analysis of factors related to abnormal liver function in patients with non alcoholic fatty liver disease[J]. Clin J Med Offic, 2025, 53( 5): 522- 524, 528. DOI: 10.16680/j.1671-3826.2025.05.21.张月霞, 王宇, 尤丛蕾, 等. 非酒精性脂肪肝患者肝功能异常相关因素分析[J]. 临床军医杂志, 2025, 53( 5): 522- 524, 528. DOI: 10.16680/j.1671-3826.2025.05.21. [5] CHEN ZB, HUANG LY, WANG BY, et al. Research advances in the association between metabolic associated fatty liver and type 2 diabetes mellitus and the mechanism of comorbidity[J]. J Clin Hepatol, 2023, 39( 10): 2454- 2459. DOI: 10.3969/j.issn.1001-5256.2023.10.025.陈兆斌, 黄丽媛, 王炳元, 等. 代谢相关脂肪性肝病与2型糖尿病的关系及共病机制研究进展[J]. 临床肝胆病杂志, 2023, 39( 10): 2454- 2459. DOI: 10.3969/j.issn.1001-5256.2023.10.025. [6] OU FB, LUO SH, LI XF, et al. Metabolic-associated fatty liver disease as a driver of chronic kidney disease[J]. J Clin Exp Med, 2023, 22( 4): 443- 447. DOI: 10.3969/j.issn.1671-4695.2023.04.030.欧芳波, 罗沈晖, 李学锋, 等. 代谢相关脂肪性肝病:慢性肾脏病的一个启动因素[J]. 临床和实验医学杂志, 2023, 22( 4): 443- 447. DOI: 10.3969/j.issn.1671-4695.2023.04.030. [7] GAO LL, WANG Y, YAN HF, et al. Characteristics of cardiometabolic risk in patients with different subtypes of non-alcoholic fatty liver disease[J]. J Clin Hepatol, 2025, 41( 1): 63- 68. DOI: 10.12449/JCH250110.高黎黎, 王勇, 严华芳, 等. 不同亚型非酒精性脂肪性肝病患者代谢性心血管病风险因素的特征分析[J]. 临床肝胆病杂志, 2025, 41( 1): 63- 68. DOI: 10.12449/JCH250110. [8] LI X, CHEN Y, LIU X, et al. Differences in the diagnosis and treatment guidelines at home and abroad for non-alcoholic fatty liver disease and treatment prospects[J]. Chin J Arterioscler, 2024, 32( 4): 347- 354. DOI: 10.20039/j.cnki.1007-3949.2024.04.010.黎翔, 陈弋, 刘霞, 等. 非酒精性脂肪性肝病国内外诊疗指南的区别及治疗展望[J]. 中国动脉硬化杂志, 2024, 32( 4): 347- 354. DOI: 10.20039/j.cnki.1007-3949.2024.04.010. [9] KAN YL, LI YM, TANG MH, et al. A prospective cohort study on the association between blood lipid levels and the risk of non-alcoholic fatty liver disease in a community population[J]. Chin Prev Med, 2024, 25( 5): 519- 525. DOI: 10.16506/j.1009-6639.2024.05.002.阚云龙, 李咏梅, 唐敏华, 等. 社区人群血脂水平与非酒精性脂肪肝病发病风险队列研究[J]. 中国预防医学杂志, 2024, 25( 5): 519- 525. DOI: 10.16506/j.1009-6639.2024.05.002. [10] DOBIÁS̆OVÁ M, FROHLICH J. The plasma parameter log(TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma(FERHDL)[J]. Clin Biochem, 2001, 34( 7): 583- 588. DOI: 10.1016/S0009-9120(01)00263-6. [11] ISMAIEL A, CIOBANU OS, ISMAIEL M, et al. Atherogenic index of plasma in non-alcoholic fatty liver disease: Systematic review and meta-analysis[J]. Biomedicines, 2022, 10( 9): 2101. DOI: 10.3390/biomedicines10092101. [12] YUAN Y, SHI J, SUN W, et al. The positive association between the atherogenic index of plasma and the risk of new-onset hypertension: A nationwide cohort study in China[J]. Clin Exp Hypertens, 2024, 46( 1): 2303999. DOI: 10.1080/10641963.2024.2303999. [13] ZHANG W, XIE WC, XU JE. Cross-sectional study of relationship between the Atherogenic Index of Plasma(AIP) and the risk of diabetes[J]. Mod Prev Med, 2018, 45( 14): 2676- 2679.张文, 解为慈, 徐金娥. 血浆致动脉硬化指数(AIP)与糖尿病关系的横断面研究[J]. 现代预防医学, 2018, 45( 14): 2676- 2679. [14] FERNÁNDEZ-MACÍAS JC, OCHOA-MARTÍNEZ AC, VARELA-SILVA JA, et al. Atherogenic index of plasma: Novel predictive biomarker for cardiovascular illnesses[J]. Arch Med Res, 2019, 50( 5): 285- 294. DOI: 10.1016/j.arcmed.2019.08.009. [15] YUAN Y, HU JW, WANG Y, et al. Association between atherogenic index of plasma and subclinical renal damage over a 12-year follow-up: Hanzhong adolescent hypertension study[J]. Eur J Clin Nutr, 2020, 74( 2): 278- 284. DOI: 10.1038/s41430-019-0530-x. [16] LIU SH, LIU Q, HAN X, et al. Association of trajectories of atherogenic index of plasma with atherosclerotic cardiovascular disease[J]. Chin Circ J, 2024, 39( 7): 676- 681. DOI: 10.3969/j.issn.1000-3614.2024.07.004.刘士贺, 刘倩, 韩旭, 等. 血浆致动脉粥样硬化指数轨迹与动脉粥样硬化性心血管疾病的关联[J]. 中国循环杂志, 2024, 39( 7): 676- 681. DOI: 10.3969/j.issn.1000-3614.2024.07.004. [17] HOU QQ, QI Q, HAN QL, et al. Association of the triglyceride-glucose index with early-onset atherosclerotic cardiovascular disease events and all-cause mortality: A prospective cohort study[J]. Cardiovasc Diabetol, 2024, 23( 1): 149. DOI: 10.1186/s12933-024-02249-4. [18] ZHANG YJ, CHEN SH, TIAN X, et al. Association between cumulative atherogenic index of plasma exposure and risk of myocardial infarction in the general population[J]. Cardiovasc Diabetol, 2023, 22( 1): 210. DOI: 10.1186/s12933-023-01936-y. [19] Fatty Liver and Alcoholic Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for management of nonalcoholic fatty liver disease: An updated and revised edition[J]. Chin J Gastroenterol Hepatol, 2010, 19( 6): 483- 487. DOI: 10.3969/j.issn.1006-5709.2010.06.001.中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南(2010年修订版)[J]. 胃肠病学和肝病学杂志, 2010, 19( 6): 483- 487. DOI: 10.3969/j.issn.1006-5709.2010.06.001. [20] Revision Committee of Chinese Guidelines for the Prevention and Treatment of Hypertension, Hypertension Alliance(China); Chinese Society of Cardiology of the Chinese Medical Association; Hypertension Committee of the Chinese Medical Doctor Association, et al. 2018 Chinese guidelines for the management of hypertension[J]. Chin J Cardiovasc Med, 2019, 24( 1): 24- 56. DOI: 10.3969/j.issn.1009-816X.2019.01.001.中国高血压防治指南修订委员会高血压联盟中国, 中华医学会心血管病学分会, 中国医师协会高血压专业委员会, 等. 中国高血压防治指南(2018年修订版)[J]. 中国心血管杂志, 2019, 24( 1): 24- 56. DOI: 10.3969/j.issn.1009-816X.2019.01.001. [21] Chinese Diabetes Society, Chinese Medical Association. Guideline for the prevention and treatment of type 2 diabetes mellitus in China(2020 edition)[J]. Chin J Diabetes Mellit, 2021, 13( 4): 315- 409. DOI: 10.3760/cma.j.cn115791-20210221-00095.中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13( 4): 315- 409. DOI: 10.3760/cma.j.cn115791-20210221-00095. [22] Joint Committee on the Chinese Guidelines for Lipid Management. Chinese guidelines for lipid management(2023)[J]. Chin Circ J, 2023, 38( 3): 237- 271. DOI: 10.3969/j.issn.1000-3614.2023.03.001.中国血脂管理指南修订联合专家委员会. 中国血脂管理指南(2023年)[J]. 中国循环杂志, 2023, 38( 3): 237- 271. DOI: 10.3969/j.issn.1000-3614.2023.03.001. [23] QIAO X, HU YQ, FAN YZ, et al. Associations between obesity and non-alcoholic fatty liver disease and the mediating role of hyperuricemia in the occupational population[J]. Mod Prev Med, 2024, 51( 4): 607- 612. DOI: 10.20043/j.cnki.MPM.202312222.乔栩, 胡玉麒, 范云喆, 等. 职业人群肥胖与非酒精性脂肪肝关联及高尿酸血症的中介作用分析[J]. 现代预防医学, 2024, 51( 4): 607- 612. DOI: 10.20043/j.cnki.MPM.202312222. [24] WANG Q, ZHENG DM, LIU J, et al. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: A cross-sectional study[J]. Lipds Health Dis, 2018, 17( 1): 284. DOI: 10.1186/s12944-018-0932-0. [25] AMARAPURKAR D, KAMANI P, PATEL N, et al. Prevalence of non-alcoholic fatty liver disease: Population based study[J]. Ann Hepatol, 2007, 6( 3): 161- 163. DOI: 10.1016/S1665-2681(19)31922-2. [26] LI KM, LI J, CHENG XY, et al. Association between the atherogenic index of plasma and new-onset non-alcoholic fatty liver disease in non-obese participants[J]. Front Endocrinol, 2022, 13: 969783. DOI: 10.3389/fendo.2022.969783. [27] PENG HW, ZHANG JC, HUANG XH, et al. Development and validation of an online dynamic nomogram based on the atherogenic index of plasma to screen nonalcoholic fatty liver disease[J]. Lipids Health Dis, 2023, 22: 44. DOI: 10.1186/s12944-023-01808-0. [28] WANG LL, YI JY, GUO XL, et al. Associations between life’s essential 8 and non-alcoholic fatty liver disease among US adults[J]. J Transl Med, 2022, 20( 1): 616. DOI: 10.1186/s12967-022-03839-0. [29] ZHANG XL, ZHENG QR, WANG QS, et al. Summary of the best evidence of exercise management for patients with nonalcoholic fatty liver disease[J]. Chin J Nurs, 2023, 58( 20): 2464- 2471. DOI: 10.3761/j.issn.0254-1769.2023.20.005.张雪玲, 郑奇容, 王巧松, 等. 非酒精性脂肪肝患者运动管理的最佳证据总结[J]. 中华护理杂志, 2023, 58( 20): 2464- 2471. DOI: 10.3761/j.issn.0254-1769.2023.20.005. [30] ZHU XR, LI X, ZHAO X, et al. Association between dietary patterns and non-alcoholic fatty liver disease in Chengdu[J]. Mod Prev Med, 2023, 50( 9): 1589- 1593, 1598. DOI: 10.20043/j.cnki.MPM.202212337.朱兴任, 李选, 赵星, 等. 成都地区膳食模式与非酒精性脂肪肝的关联性研究[J]. 现代预防医学, 2023, 50( 9): 1589- 1593, 1598. DOI: 10.20043/j.cnki.MPM.202212337. [31] YANG R, WANG L. Influence of lifestyle habits on nonalcoholic fatty liver disease[J]. Sci Sin Vitae, 2024, 54( 5): 819- 830. DOI: 10.1360/SSV-2024-0023.杨睿, 王琳. 生活习惯对非酒精性脂肪肝病的影响[J]. 中国科学: 生命科学, 2024, 54( 5): 819- 830. DOI: 10.1360/SSV-2024-0023. -



 PDF下载 ( 1387 KB)
PDF下载 ( 1387 KB)

 下载:
下载:


