肝纤维化治疗药物的研究进展
DOI: 10.12449/JCH251027
-
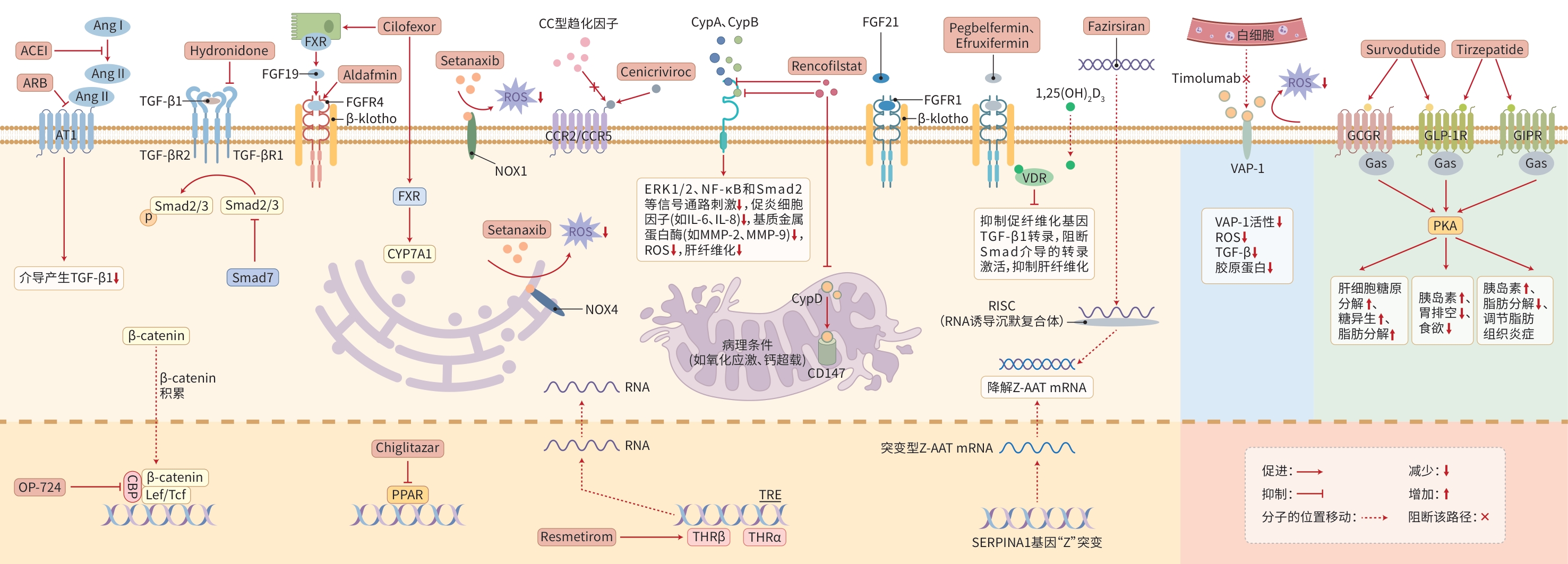
摘要: 肝纤维化是以肝星状细胞活化、细胞外基质过度沉积为主要特征的一种慢性病理状态,随着肝纤维化不断进展,可导致肝硬化甚至肝细胞癌,因此,逆转肝纤维化对提高患者的生存质量、延长生存时间有着重要意义。目前已有多种肝纤维化的治疗药物进入临床试验阶段。本文对近年来肝纤维化治疗相关药物的研究进展进行综述,以期为肝纤维化治疗及未来药物研究方向提供思路。Abstract: Hepatic fibrosis is a chronic pathological condition characterized by hepatic stellate cell activation and excessive deposition of extracellular matrix, which would progress to liver cirrhosis and even hepatocellular carcinoma. Therefore, reversal of hepatic fibrosis is of great importance for improving quality of life and prolonging survival time. Currently, various therapeutic drugs for hepatic fibrosis have entered the stage of clinical trial. This article reviews the research advances in therapeutic drugs for hepatic fibrosis in the recent years, in order to provide insights into the treatment of hepatic fibrosis and future research directions for drugs.
-
Key words:
- Hepatic Fibrosis /
- Drug Therapy /
- Treatment Outcome
-
表 1 处于临床试验阶段的肝纤维化治疗药物
Table 1. Hepatic fibrosis therapeutic agents in clinical trials
药物类别 药物 受试者 试验注册号 研究阶段 参考文献 进展情况 RAS抑制剂 Ramipril或
CandesartanCHC肝纤维化 NCT03770936 Ⅲ期 [8] 已上市 TGF-β1抑制剂 Hydronidone CHB肝纤维化 NCT02499562 Ⅱ期 [10] 已完成 NCT05905172 Ⅲb期 临床试验 FGF19类似物 Aldafermin NASH代偿期肝硬化 NCT04210245 Ⅱb期 [14] 已完成 NASH F2/F3期肝纤维化 NCT03912532 Ⅱb期 [15] 已完成 FXR激动剂 Cilofexor PSC非肝硬化 NCT02943460 Ⅱ期 [16] 临床试验 泛PPAR激动剂 Chiglitazar MASLD肝硬化 NCT06773221 筹备中 CCR2和CCR5受体
拮抗剂Cenicriviroc MASH F1~F3期肝纤维化 NCT03059446 Ⅱ期 [19] 提前终止 CBP/β-catenin抑制剂 OP-724 HIV/HCV共感染引起的
血友病合并肝硬化NCT04688034 Ⅰ期 [23] 临床试验 Cyp抑制剂 Rencofilstat NASH F2/F3期肝纤维化 NCT04480710 Ⅱa期 [26] 已完成 NASH晚期肝纤维化 NCT05402371 筹备中 VAP-1抗体 Timolumab PSC肝纤维化 NCT02239211 Ⅱ期 [28] 临床试验 NOX1/4抑制剂 Setanaxib PBC肝纤维化 NCT03226067 Ⅱ期 [30] 临床试验 利福平衍生物 Rifaximin-α 酒精相关性肝病 2014-001856-51 Ⅱ期 [32] 已上市 磺胺类抗菌药 Sulfasalazine PBC、乙型和丙型肝炎
肝硬化以及酒精性肝硬化NCT06293378 临床试验 益生菌制剂 嗜酸乳杆菌+
乳双歧杆菌NASH NCT02764047 [35] 已上市 肝细胞靶向的RNA
干扰治疗药物Fazirsiran AAT缺乏症相关肝纤维化 NCT03945292 Ⅱ期 [36] 已完成 NCT05677971 Ⅲ期 临床试验 FGF21的聚乙二醇
缀合类似物Pegbelfermin NASH F3期肝纤维化 NCT03486899 Ⅱb期 [37] 临床试验 二价Fc-FGF21类似物 Efruxifermin NASH F2/F3期肝纤维化 NCT04767529 Ⅱb期 [38] 临床试验 THR-β选择性激动剂 Resmetirom NASH NCT03900429 Ⅲ期 [39] 已上市 GCGR和GLP-1R的
激动剂Tirzepatide MASH F2/F3期肝纤维化 NCT04166773 Ⅱ期 [40] 已上市 GLP-1类似物 Semaglutide NASH代偿期肝硬化 NCT03987451 Ⅱ期 [41] 已上市 GIPR和GLP-1R的
单分子双重激动剂Survodutide MASH肝硬化 NCT05296733 Ⅰ期 [42] 已完成 MASH肝纤维化 NCT04771273 Ⅱ期 [43] 已完成 中药制剂 鳖甲煎丸 CHB肝纤维化/肝硬化 ChiCTR1800016801 [34] 已上市 安络化纤丸 未进行抗病毒治疗的CHB ChiCTR-IOR-14005474 [53] 已上市 软肝颗粒 CHB晚期肝纤维化/早期
肝硬化ChiCTR-IIR-15007567 [55] 已完成 注:GCGR,葡萄糖依赖性促胰岛素多肽受体;GLP-1R,胰高血糖素样肽-1受体;GIPR,胰高血糖素受体。
-
[1] MOON AM, SINGAL AG, TAPPER EB. Contemporary epidemiology of chronic liver disease and cirrhosis[J]. Clin Gastroenterol Hepatol, 2020, 18( 12): 2650- 2666. DOI: 10.1016/j.cgh.2019.07.060. [2] BERUMEN J, BAGLIERI J, KISSELEVA T, et al. Liver fibrosis: Pathophysiology and clinical implications[J]. WIREs Mech Dis, 2021, 13( 1): e1499. DOI: 10.1002/wsbm.1499. [3] LIAO ZH, XIE ZY. Research progress in molecular mechanism of hepatic fibrosis and related therapeutic targets[J]. J Jilin Univ(Med Edit), 2024, 50( 5): 1450- 1456. DOI: 10.13481/j.1671-587X.20240532.廖昭辉, 谢正元. 肝纤维化发病的分子机制及其相关治疗靶点的研究进展[J]. 吉林大学学报(医学版), 2024, 50( 5): 1450- 1456. DOI: 10.13481/j.1671-587X.20240532. [4] BOGOMOLOV PO, IVASHKIN VT, BUEVEROV AO, et al. Efficacy and safety of bulevirtide in patients with chronic hepatitis D and compensated cirrhosis[J]. Ter Arkh, 2021, 93( 11): 1290- 1299. DOI: 10.26442/00403660.2021.11.201163. [5] CHO Y, RHEE H, KIM YE, et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: An open-label randomized controlled trial(ESSENTIAL study)[J]. BMC Med, 2022, 20( 1): 93. DOI: 10.1186/s12916-022-02288-2. [6] HOOSHMAND GHARABAGH L, SHARGH A, MOHAMMAD HOSSEINI AZAR MR, et al. Comparison between the effect of Empagliflozin and Pioglitazone added to metformin in patients with type 2 diabetes and nonalcoholic fatty liver disease[J]. Clin Res Hepatol Gastroenterol, 2024, 48( 3): 102279. DOI: 10.1016/j.clinre.2023.102279. [7] LI X, MENG Y, YANG XS, et al. ACEI attenuates the progression of CCl4-induced rat hepatic fibrogenesis by inhibiting TGF-beta1, PDGF-BB, NF-kappaB and MMP-2, 9[J]. World J Gastroenterol, 2005, 11( 31): 4807- 4811. DOI: 10.3748/wjg.v11.i31.4807. [8] MOSTAFA TM, EL-AZAB GA, BADRA GA, et al. Effect of candesartan and ramipril on liver fibrosis in patients with chronic hepatitis C viral infection: A randomized controlled prospective study[J]. Curr Ther Res Clin Exp, 2021, 95: 100654. DOI: 10.1016/j.curtheres.2021.100654. [9] WANG ML, ZHANG QD, QU Y, et al. Experimental study of hydronidone in the treatment of hepatic fibrosis induced by carbon tetrachloride in mice[J]. Int J Dig Dis, 2019, 39( 3): 175- 180, 231. DOI: 10.3969/j.issn.1673-534X.2019.03.008.王美玲, 张启迪, 曲颖, 等. 羟尼酮治疗四氯化碳诱导小鼠肝纤维化的实验研究[J]. 国际消化病杂志, 2019, 39( 3): 175- 180, 231. DOI: 10.3969/j.issn.1673-534X.2019.03.008. [10] CAI XB, LIU XH, XIE W, et al. Hydronidone for the treatment of liver fibrosis related to chronic hepatitis B: A phase 2 randomized controlled trial[J]. Clin Gastroenterol Hepatol, 2023, 21( 7): 1893- 1901. DOI: 10.1016/j.cgh.2022.05.056. [11] BARCHETTA I, CIMINI FA, CAVALLO MG. Vitamin D and metabolic dysfunction-associated fatty liver disease(MAFLD): An update[J]. Nutrients, 2020, 12( 11): 3302. DOI: 10.3390/nu12113302. [12] EBRAHIMPOUR-KOUJAN S, SOHRABPOUR AA, GIOVANNUCCI E, et al. Effects of vitamin D supplementation on liver fibrogenic factors, vitamin D receptor and liver fibrogenic microRNAs in metabolic dysfunction-associated steatotic liver disease(MASLD) patients: An exploratory randomized clinical trial[J]. Nutr J, 2024, 23( 1): 24. DOI: 10.1186/s12937-024-00911-x. [13] CHIANG JYL, FERRELL JM. Discovery of farnesoid X receptor and its role in bile acid metabolism[J]. Mol Cell Endocrinol, 2022, 548: 111618. DOI: 10.1016/j.mce.2022.111618. [14] RINELLA ME, LIEU HD, KOWDLEY KV, et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis[J]. Hepatology, 2024, 79( 3): 674- 689. DOI: 10.1097/HEP.0000000000000607. [15] HARRISON SA, ABDELMALEK MF, NEFF G, et al. Aldafermin in patients with non-alcoholic steatohepatitis(ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial[J]. Lancet Gastroenterol Hepatol, 2022, 7( 7): 603- 616. DOI: 10.1016/S2468-1253(22)00017-6. [16] TRAUNER M, BOWLUS CL, GULAMHUSEIN A, et al. Safety and sustained efficacy of the farnesoid X receptor(FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC[J]. Clin Gastroenterol Hepatol, 2023, 21( 6): 1552- 1560. DOI: 10.1016/j.cgh.2022.07.024. [17] QIU YY, ZHANG J, ZENG FY, et al. Roles of the peroxisome proliferator-activated receptors(PPARs) in the pathogenesis of nonalcoholic fatty liver disease(NAFLD)[J]. Pharmacol Res, 2023, 192: 106786. DOI: 10.1016/j.phrs.2023.106786. [18] WANG YM, LI HQ, GAO H, et al. Effect of chiglitazar and sitagliptin on glucose variations, insulin resistance and inflammatory-related biomarkers in untreated patients with type 2 diabetes[J]. Diabetes Res Clin Pract, 2022, 183: 109171. DOI: 10.1016/j.diabres.2021.109171. [19] FRANCQUE SM, HODGE A, BOURSIER J, et al. Phase 2, open-label, rollover study of cenicriviroc for liver fibrosis associated with metabolic dysfunction-associated steatohepatitis[J]. Hepatol Commun, 2024, 8( 2): e0335. DOI: 10.1097/HC9.0000000000000335. [20] OUCHI H, MIZUTANI Y, YOSHIMURA K, et al. Anti-inflammatory and antifibrotic effects of CBP/β-catenin inhibitor for hepatocytes: Small molecular inhibitor, OP-724 possibly improves liver function[J]. Med Mol Morphol, 2023, 56( 2): 94- 105. DOI: 10.1007/s00795-022-00343-8. [21] KIMURA K, KANTO T, SHIMODA S, et al. Safety, tolerability, and anti-fibrotic efficacy of the CBP/β-catenin inhibitor PRI-724 in patients with hepatitis C and B virus-induced liver cirrhosis: An investigator-initiated, open-label, non-randomised, multicentre, phase 1/2a study[J]. EBioMedicine, 2022, 80: 104069. DOI: 10.1016/j.ebiom.2022.104069. [22] KIMURA M, OGAWA E, HARADA K, et al. Feasibility, safety and tolerability of the CREB-binding protein/β-catenin inhibitor OP-724 in patients with advanced primary biliary cholangitis: An investigator-initiated, open-label, non-randomised, two-centre, phase 1 study[J]. BMJ Open Gastroenterol, 2022, 9( 1): e001001. DOI: 10.1136/bmjgast-2022-001001. [23] KIMURA K, TANUMA J, KIMURA M, et al. Safety and tolerability of OP-724 in patients with haemophilia and liver cirrhosis due to HIV/HCV coinfection: An investigator-initiated, open-label, non-randomised, single-centre, phase I study[J]. BMJ Open Gastroenterol, 2024, 11( 1): e001341. DOI: 10.1136/bmjgast-2023-001341. [24] URE DR, TREPANIER DJ, MAYO PR, et al. Cyclophilin inhibition as a potential treatment for nonalcoholic steatohepatitis(NASH)[J]. Expert Opin Investig Drugs, 2020, 29( 2): 163- 178. DOI: 10.1080/13543784.2020.1703948. [25] KUO J, BOBARDT M, CHATTERJI U, et al. A pan-cyclophilin inhibitor, CRV431, decreases fibrosis and tumor development in chronic liver disease models[J]. J Pharmacol Exp Ther, 2019, 371( 2): 231- 241. DOI: 10.1124/jpet.119.261099. [26] HARRISON SA, MAYO PR, HOBBS TM, et al. Rencofilstat, a cyclophilin inhibitor: A phase 2a, multicenter, single-blind, placebo-controlled study in F2/F3 NASH[J]. Hepatol Commun, 2022, 6( 12): 3379- 3392. DOI: 10.1002/hep4.2100. [27] WESTON CJ, SHEPHERD EL, CLARIDGE LC, et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis[J]. J Clin Invest, 2015, 125( 2): 501- 520. DOI: 10.1172/JCI73722. [28] HIRSCHFIELD GM, ARNDTZ K, KIRKHAM A, et al. Vascular adhesion protein-1 blockade in primary sclerosing cholangitis: Open-label, multicenter, single-arm, phase II trial[J]. Hepatol Commun, 2024, 8( 5): e0426. DOI: 10.1097/HC9.0000000000000426. [29] AOYAMA T, PAIK YH, WATANABE S, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent[J]. Hepatology, 2012, 56( 6): 2316- 2327. DOI: 10.1002/hep.25938. [30] INVERNIZZI P, CARBONE M, JONES D, et al. Setanaxib, a first-in-class selective NADPH oxidase 1/4 inhibitor for primary biliary cholangitis: A randomized, placebo-controlled, phase 2 trial[J]. Liver Int, 2023, 43( 7): 1507- 1522. DOI: 10.1111/liv.15596. [31] MASLENNIKOV R, POLUEKTOVA E, ZOLNIKOVA O, et al. Gut microbiota and bacterial translocation in the pathogenesis of liver fibrosis[J]. Int J Mol Sci, 2023, 24( 22): 16502. DOI: 10.3390/ijms242216502. [32] ISRAELSEN M, MADSEN BS, TORP N, et al. Rifaximin-α for liver fibrosis in patients with alcohol-related liver disease(GALA-RIF): A randomised, double-blind, placebo-controlled, phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2023, 8( 6): 523- 532. DOI: 10.1016/S2468-1253(23)00010-9. [33] BAI GP, ZHANG RH, YAN GH, et al. The effect of modified turtle shell decoction on the expression of TGFβ1, Smad3 and Smad7 in rat hepatic stellate cell[J]. Immunol J, 2017, 33( 9): 777- 782. DOI: 10.13431/j.cnki.immunol.j.20170137.柏干苹, 张荣华, 闫国和, 等. 鳖甲煎改良方对肝星状细胞生长及TGFβ1、Smad3与Smad7表达的影响[J]. 免疫学杂志, 2017, 33( 9): 777- 782. DOI: 10.13431/j.cnki.immunol.j.20170137. [34] CHI X, CHENG DY, SUN X, et al. Efficacy of Biejiajian pill on intestinal microbiota in patients with hepatitis B cirrhosis/liver fibrosis: A randomized double-blind controlled trial[J]. Chin J Integr Med, 2023, 29( 9): 771- 781. DOI: 10.1007/s11655-023-3542-2. [35] ESCOUTO GS, PORT GZ, TOVO CV, et al. Probiotic supplementation, hepatic fibrosis, and the microbiota profile in patients with nonalcoholic steatohepatitis: A randomized controlled trial[J]. J Nutr, 2023, 153( 7): 1984- 1993. DOI: 10.1016/j.tjnut.2023.05.019. [36] CLARK VC, STRANGE C, STRNAD P, et al. Fazirsiran for adults with alpha-1 antitrypsin deficiency liver disease: A phase 2 placebo controlled trial(SEQUOIA)[J]. Gastroenterology, 2024, 167( 5): 1008- 1018. DOI: 10.1053/j.gastro.2024.06.028. [37] LOOMBA R, SANYAL AJ, NAKAJIMA A, et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and stage 3 fibrosis(FALCON 1): A randomized phase 2b study[J]. Clin Gastroenterol Hepatol, 2024, 22( 1): 102- 112. DOI: 10.1016/j.cgh.2023.04.011. [38] HARRISON SA, FRIAS JP, NEFF G, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis(HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial[J]. Lancet Gastroenterol Hepatol, 2023, 8( 12): 1080- 1093. DOI: 10.1016/S2468-1253(23)00272-8. [39] HARRISON SA, BEDOSSA P, GUY CD, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis[J]. N Engl J Med, 2024, 390( 6): 497- 509. DOI: 10.1056/NEJMoa2309000. [40] LOOMBA R, HARTMAN ML, LAWITZ EJ, et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis[J]. N Engl J Med, 2024, 391( 4): 299- 310. DOI: 10.1056/NEJMoa2401943. [41] LOOMBA R, ABDELMALEK MF, ARMSTRONG MJ, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2023, 8( 6): 511- 522. DOI: 10.1016/S2468-1253(23)00068-7. [42] LAWITZ EJ, FRAESSDORF M, NEFF GW, et al. Efficacy, tolerability and pharmacokinetics of survodutide, a glucagon/glucagon-like peptide-1 receptor dual agonist, in cirrhosis[J]. J Hepatol, 2024, 81( 5): 837- 846. DOI: 10.1016/j.jhep.2024.06.003. [43] SANYAL AJ, BEDOSSA P, FRAESSDORF M, et al. A phase 2 randomized trial of survodutide in MASH and fibrosis[J]. N Engl J Med, 2024, 391( 4): 311- 319. DOI: 10.1056/NEJMoa2401755. [44] PERVA IT, SIMINA IE, BENDE R, et al. Use of a micronutrient cocktail to improve metabolic dysfunction-associated steatotic liver disease(MASLD) in adults with obesity: A randomized, double-blinded pilot clinical trial[J]. Medicina(Kaunas), 2024, 60( 8): 1366. DOI: 10.3390/medicina60081366. [45] ARMANDI A, BESPALJKO H, MANG A, et al. Short-term reduction of dietary gluten improves metabolic-dysfunction associated steatotic liver disease: A randomised, controlled proof-of-concept study[J]. Aliment Pharmacol Ther, 2024, 59( 10): 1212- 1222. DOI: 10.1111/apt.17941. [46] SILVA-CARVALHO R, BALTAZAR F, ALMEIDA-AGUIAR C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development[J]. Evid Based Complement Alternat Med, 2015, 2015: 206439. DOI: 10.1155/2015/206439. [47] NIKBAF-SHANDIZ M, TUTUNCHI H, KHOSHBATEN M, et al. Propolis supplementation in obese patients with non-alcoholic fatty liver disease: Effects on glucose homeostasis, lipid profile, liver function, anthropometric indices and meta-inflammation[J]. Food Funct, 2022, 13( 22): 11568- 11578. DOI: 10.1039/d2fo01280d. [48] WU LC, HO JA, SHIEH MC, et al. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts[J]. J Agric Food Chem, 2005, 53( 10): 4207- 4212. DOI: 10.1021/jf0479517. [49] STINE JG, WELLES JE, KEATING S, et al. Serum fibroblast growth factor 21 is markedly decreased following exercise training in patients with biopsy-proven nonalcoholic steatohepatitis[J]. Nutrients, 2023, 15( 6): 1481. DOI: 10.3390/nu15061481. [50] HARRIS SJ, SMITH N, HUMMER B, et al. Exercise training improves serum biomarkers of liver fibroinflammation in patients with metabolic dysfunction-associated steatohepatitis[J]. Liver Int, 2024, 44( 2): 532- 540. DOI: 10.1111/liv.15769. [51] GUO XL, JIA ZS, ZHANG J. Molecular mechanisms of traditional Chinese medicine in reversing liver fibrosis[J]. J Clin Hepatol, 2025, 41( 1): 170- 175. DOI: 10.12449/JCH250126.郭晓玲, 贾战生, 张静. 中药逆转肝纤维化的分子机制[J]. 临床肝胆病杂志, 2025, 41( 1): 170- 175. DOI: 10.12449/JCH250126. [52] LI MQ, LIU FR, GUO XJ, et al. Clinical observation of Kucai paste combined with Entecavir in the treatment of chronic hepatitis B liver fibrosis[J]. J Changchun Univ Chin Med, 2024, 40( 3): 301- 305. DOI: 10.13463/j.cnki.cczyy.2024.03.015.李梦琪, 刘繁荣, 郭新建, 等. 苦菜膏联合恩替卡韦治疗慢性乙型肝炎肝纤维化的临床观察[J]. 长春中医药大学学报, 2024, 40( 3): 301- 305. DOI: 10.13463/j.cnki.cczyy.2024.03.015. [53] Liver Disease Committee, Chinese Association of Integrative Medicine. Guidelines for the diagnosis and treatment of liver fibrosis in integrative medicine practice(2019)[J]. J Clin Hepatol, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007.中国中西医结合学会肝病专业委员会. 肝纤维化中西医结合诊疗指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007. [54] XIAO HM, SHI MJ, JIANG JM, et al. Efficacy and safety of AnluoHuaxian pills on chronic hepatitis B with normal or minimally elevated alanine transaminase and early liver fibrosis: A randomized controlled trial[J]. J Ethnopharmacol, 2022, 293: 115210. DOI: 10.1016/j.jep.2022.115210. [55] PENG DT, XING YF, CHEN L, et al. Clinical study of Ruangan granules combined with Entecavir in the treatment of severe hepatitis B fibrosis[J]. Lishizhen Med Mater Med Res, 2019, 30( 8): 1934- 1936. DOI: 10.3969/j.issn.1008-0805.2019.08.050.彭得倜, 邢宇锋, 陈亮, 等. 软肝颗粒联合恩替卡韦治疗乙肝重度肝纤维化的临床研究[J]. 时珍国医国药, 2019, 30( 8): 1934- 1936. DOI: 10.3969/j.issn.1008-0805.2019.08.050. [56] XING YF, ZHONG WC, PENG DT, et al. Chinese herbal formula Ruangan granule enhances the efficacy of entecavir to reverse advanced liver fibrosis/early cirrhosis in patients with chronic HBV infection: A multicenter, randomized clinical trial[J]. Pharmacol Res, 2023, 190: 106737. DOI: 10.1016/j.phrs.2023.106737. -



 PDF下载 ( 848 KB)
PDF下载 ( 848 KB)

 下载:
下载:


