Wilson病肝硬化并发肌肉减少症的危险因素及其对临床结局的影响
DOI: 10.12449/JCH251018
Risk factors for sarcopenia in patients with Wilson’s disease-related liver cirrhosis and their impact on clinical outcomes
-
摘要:
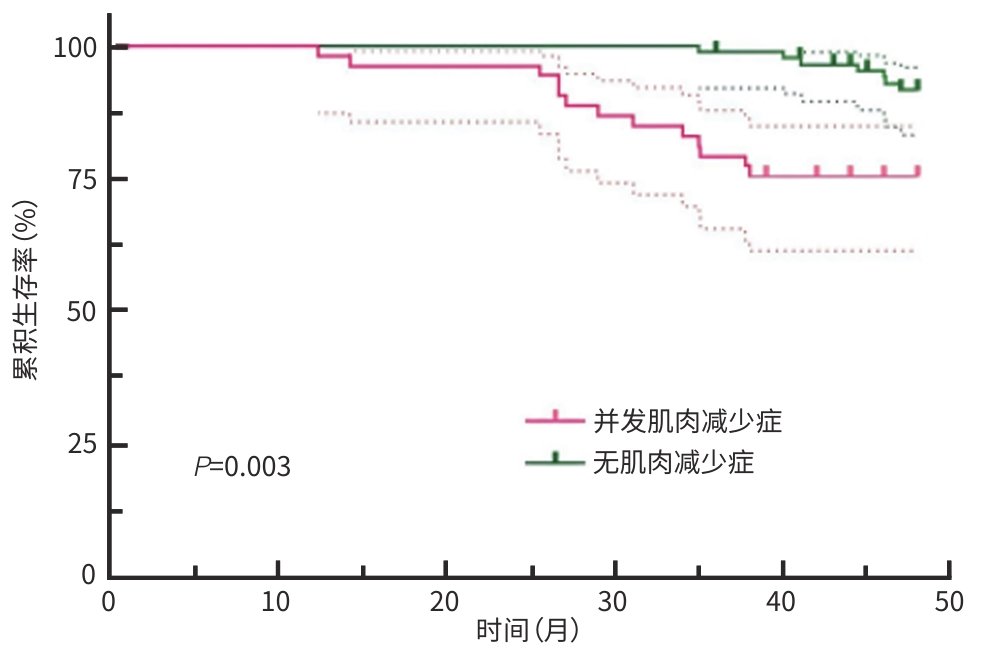
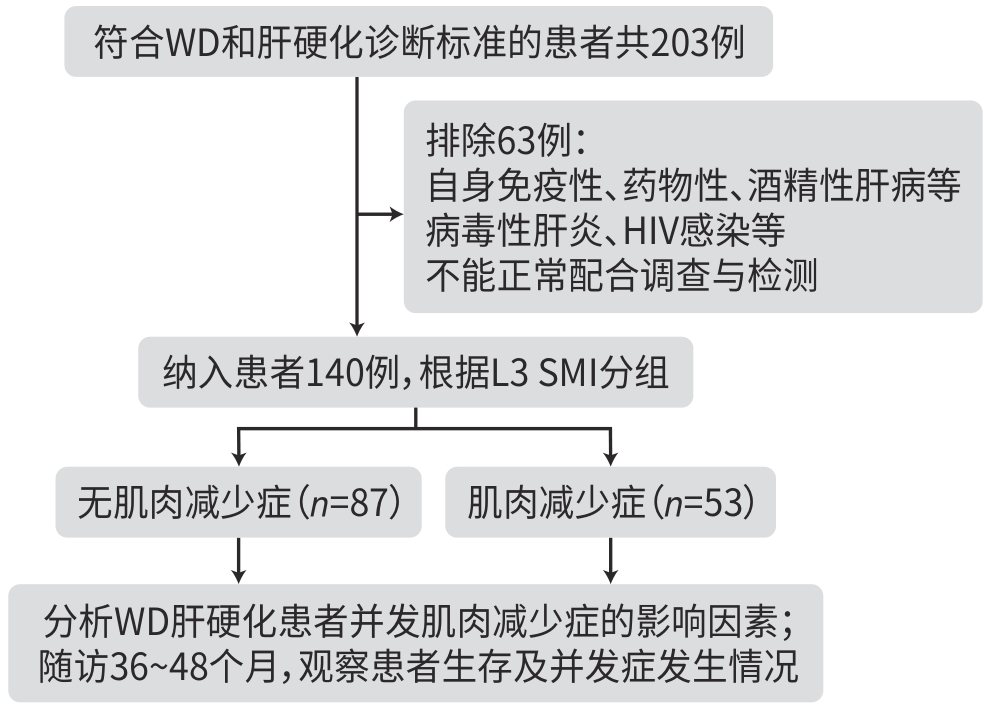
目的 研究Wilson病肝硬化患者中肌肉减少症的发生情况,探讨肌肉减少症发生的危险因素及其对临床结局的影响。 方法 纳入2019年1月—2020年6月在安徽中医药大学第一附属医院接受治疗的140例Wilson病肝硬化患者,根据第三腰椎骨骼肌质量指数(L3 SMI)将患者分为肌肉减少症组和无肌肉减少症组。对纳入患者进行营养风险筛查、人体测量、血生化指标检测,比较两组相关指标的差异,筛选并发肌肉减少症的影响因素。随访36~48个月,比较两组患者生存状况、并发症发生情况。符合正态分布的计量资料2组间比较采用成组t检验;计数资料2组间比较采用χ2检验或Mann-Whitney U秩和检验。采用二元Logistic回归分析肌肉减少症的影响因素;通过单因素及多因素Cox回归分析影响Wilson病肝硬化患者预后的危险因素,绘制Kaplan-Meier生存曲线,采用Log-rank检验比较组间生存情况。 结果 Wilson病肝硬化中并发肌肉减少症患者53例(37.9%),其身体质量指数(BMI)和L3 SMI明显低于无肌肉减少症患者(t值分别为10.550、3.982,P值均<0.001)。Logistic多因素回归分析结果显示,Wilson病肝硬化患者并发肌肉减少症的主要影响因素为年龄(OR=2.243,95%CI:1.196~4.208,P=0.012)、性别(OR=0.450,95%CI:0.232~0.872,P=0.018)、BMI(OR=0.126,95%CI:0.089~0.294,P<0.001)、肝性脑病(OR=8.367,95%CI:2.423~28.897,P<0.001)。并发肌肉减少症患者的病死率(χ2=6.158,P=0.019)以及感染(χ2=8.008,P=0.040)、反复腹/胸腔积液(χ2=17.742,P<0.001)、肝性脑病(χ2=4.338,P=0.039)的发生率均高于无肌肉减少症者,差异均有统计学意义。多因素Cox回归分析显示,肌肉减少症(HR=4.685,P=0.002)和肝性脑病(HR=19.156,P<0.001)为影响Wilson病肝硬化患者死亡的独立危险因素。Kaplan-Meier生存曲线提示,并发肌肉减少症的患者生存率显著下降(P=0.003)。 结论 肌肉减少症是Wilson病肝硬化患者营养不良的表现之一,其病死率、其他并发症的发生风险升高,对预后产生不良影响。男性患者、并发肝性脑病、BMI水平越低、年龄越大,肌肉减少症的发生风险越高。 Abstract:Objective To investigate the incidence rate of sarcopenia in patients with Wilson’s disease (WD)-related liver cirrhosis, as well as the risk factors for sarcopenia and their impact on clinical outcomes. Methods A total of 140 patients with WD-related liver cirrhosis who were treated in The First Affiliated Hospital of Anhui University of Chinese Medicine from January 2019 to June 2020, and according to the third lumbar skeletal muscle mass index (L3 SMI), the patients were divided into sarcopenia group and non-sarcopenia group. Nutritional risk screening, anthropometric measurements, and blood biochemical tests were performed for the patients to identify the influencing factors for sarcopenia. The patients were followed up for 36 — 48 months, and survival status and complications were compared between the two groups. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the chi-square test and the Mann-Whitney U rank sum test were used for comparison of categorical data between two groups. A binary Logistic regression analysis was used to investigate the influencing factors for sarcopenia, and univariate and multivariate Cox regression analyses were used to investigate the risk factors for the prognosis of patients with WD-related liver cirrhosis. The Kaplan-Meier survival curve was plotted, and the Log-rank test was used for comparison between groups. Results Among the 140 patients with WD-related liver cirrhosis, 53 (37.9%) developed sarcopenia, with significantly lower body mass index (BMI) and L3 SMI than the patients without sarcopenia (t=10.550 and 3.982, both P<0.001). The multivariate Logistic regression analysis showed that age (odds ratio [OR]=2.243, 95% confidence interval [CI]: 1.196 — 4.208, P=0.012), sex (OR=0.450, 95%CI: 0.232 — 0.872, P=0.018), BMI (OR=0.126, 95%CI: 0.089 — 0.294, P<0.001), and hepatic encephalopathy (OR=8.367, 95%CI: 2.423 — 28.897, P<0.001) were the main influencing factors for sarcopenia in patients with WD-related liver cirrhosis. Compared with the non-sarcopenia group, the sarcopenia group had significantly higher mortality rate (χ2=6.158, P=0.019) and significantly higher incidence rates of infection (χ2=8.008, P=0.040), recurrent abdominal/pleural efflux (χ2=17.742, P<0.001), and hepatic encephalopathy (χ2=4.338, P=0.039). The multivariate Cox regression analysis showed that sarcopenia (hazard ratio [HR]=4.685, P=0.002) and hepatic encephalopathy (HR=19.156, P<0.001) were independent risk factors for death in patients with WD-related liver cirrhosis. The Kaplan-Meier survival curve analysis showed a significant reduction in survival rate in the patients with sarcopenia (P=0.003). Conclusion Sarcopenia is one of the manifestations of malnutrition in patients with WD-related liver cirrhosis, which increases the risk of mortality and other complications and has an adverse effect on prognosis. There is an increased risk of sarcopenia in male patients or patients with hepatic encephalopathy, a lower level of BMI or an older age. -
Key words:
- Hepatolenticular Degeneration /
- Liver Cirrhosis /
- Sarcopenia /
- Risk Factors
-
表 1 WD肝硬化并发肌肉减少症与无肌肉减少症患者相关指标比较
Table 1. Comparison of relevant indicators between the sarcopenia group and the non-sarcopenia group in WD patients complicated with liver cirrhosis
项目 无肌肉减少症(n=87) 并发肌肉减少症(n=53) 统计值 P值 年龄(岁) 36.9±11.1 45.3±11.1 t=4.340 <0.001 性别(例) χ2=0.782 0.424 男 44 31 女 43 22 NRS-2002(例) χ2=5.385 0.027 ≥3分 71 50 <3分 16 3 并发症(例) EV 76 42 χ2=2.896 0.112 胸/腹水 47 30 χ2=0.862 0.374 HE 6 12 χ2=5.213 0.027 Child-Pugh分级(例) Z=-2.699 0.007 A级 21 4 B级 37 23 C级 29 26 L3 SMI(cm²/m²) 48.5±6.1 33.7±4.2 t=10.550 <0.001 TP(g/L) 63.54±8.42 62.44±8.87 t=0.736 0.463 Alb(g/L) 34.30±4.82 33.00±5.60 t=1.459 0.147 Hb(g/L) 112.91±21.40 104.45±20.46 t=0.351 0.726 BMI(kg/m²) 24.2±4.5 21.4±3.3 t=3.982 <0.001 表 2 WD肝硬化患者并发肌肉减少症影响因素的Logistic回归分析
Table 2. Logistic regression analysis of influencing factors of Wilson’s disease cirrhosis combined with sarcopenia
自变量 β值 SE Wald OR(95%CI) P值 年龄 0.808 0.321 6.335 2.243(1.196~4.208) 0.012 性别 -0.799 0.338 5.600 0.450(0.232~0.872) 0.018 BMI -1.823 0.306 35.409 0.126(0.089~0.294) <0.001 HE 2.124 0.632 11.285 8.367(2.423~28.897) <0.001 注:性别赋值女=1,男=0;HE赋值是=1,否=0。
表 3 WD肝硬化并发肌肉减少症患者在随访期间的临床结局
Table 3. Clinical outcomes during follow-up in Wilson’s disease cirrhosis combined with sarcopenia
组别 例数 死亡[例(%)] 反复胸/腹水[例(%)] EV[例(%)] 感染[例(%)] HE[例(%)] 无肌肉减少症 87 7(8.0) 29(33.3) 50(57.5) 13(14.9) 12(13.8) 并发肌肉减少症 53 13(24.5) 29(54.7) 41(77.4) 12(22.6) 13(24.5) χ2值 6.158 17.742 1.182 8.008 4.338 P值 0.019 <0.001 0.337 0.040 0.039 表 4 WD肝硬化患者死亡影响因素的Cox回归分析
Table 4. Cox regression analysis of factors affecting mortality in patients with Wilson’s disease cirrhosis
项目 β值 SE Wald HR P值 单因素分析 肌肉减少症 4.396 1.663 6.985 81.117 0.008 年龄 1.096 0.495 4.897 2.991 0.027 性别 0.091 0.356 0.066 1.095 0.798 BMI -1.568 0.442 12.582 0.209 <0.001 HE 1.543 0.491 9.879 4.679 0.002 EV 1.106 0.554 3.984 3.022 0.046 胸/腹水 0.245 0.388 0.400 1.278 0.527 多因素分析 肌肉减少症 1.544 0.498 9.633 4.685 0.002 HE 2.953 0.554 28.360 19.156 <0.001 -
[1] SAYER AA, CRUZ-JENTOFT A. Sarcopenia definition, diagnosis and treatment: Consensus is growing[J]. Age Ageing, 2022, 51( 10): afac220. DOI: 10.1093/ageing/afac220. [2] CRUZ-JENTOFT AJ, BAHAT G, BAUER J, et al. Sarcopenia: Revised European consensus on definition and diagnosis[J]. Age Ageing, 2019, 48( 1): 16- 31. DOI: 10.1093/ageing/afy169. [3] YANG YJ, KIM DJ. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: Osteoporosis, sarcopenia, and osteoporotic sarcopenia[J]. Int J Mol Sci, 2021, 22( 5): 2604. DOI: 10.3390/ijms22052604. [4] JIANG MJ, HUA X, WU MC, et al. Longitudinal changes in sarcopenia was associated with survival among cirrhotic patients[J]. Front Nutr, 2024, 11: 1375994. DOI: 10.3389/fnut.2024.1375994. [5] LAI JC, TANDON P, BERNAL W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases[J]. Hepatology, 2021, 74( 3): 1611- 1644. DOI: 10.1002/hep.32049. [6] European Association for the Study of the Liver. EASL clinical practice guidelines: Wilson’s disease[J]. J Hepatol, 2012, 56( 3): 671- 685. DOI: 10.1016/j.jhep.2011.11.007. [7] Chinese Society of Neurogenetics. Chinese guidelines for diagnosis and treatment of Wilson’s disease 2021[J]. Chin J Neurol, 2021, 54( 4): 310- 319. DOI: 10.3760/cma.j.cn113694-20200826-00661.中华医学会神经病学分会神经遗传学组. 中国肝豆状核变性诊治指南2021[J]. 中华神经科杂志, 2021, 54( 4): 310- 319. DOI: 10.3760/cma.j.cn113694-20200826-00661. [8] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35( 11): 2408- 2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [9] CEDERHOLM T, JENSEN GL, CORREIA MITD, et al. GLIM criteria for the diagnosis of malnutrition-A consensus report from the global clinical nutrition community[J]. Clin Nutr, 2019, 38( 1): 1- 9. DOI: 10.1016/j.clnu.2018.08.002. [10] WELCH N, ATTAWAY A, BELLAR A, et al. Compound sarcopenia in hospitalized patients with cirrhosis worsens outcomes with increasing age[J]. Nutrients, 2021, 13( 2): 659. DOI: 10.3390/nu13020659. [11] NISHIKAWA H, SHIRAKI M, HIRAMATSU A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease(1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria[J]. Hepatol Res, 2016, 46( 10): 951- 963. DOI: 10.1111/hepr.12774. [12] XIN JH, GUO YQ, LIU Y, et al. Survival rate and quality of life in patients with liver cirrhosis complicated with bacterial infection[J]. J Public Health Prev Med, 2024, 35( 2): 101- 105. DOI: 10.3969/j.issn.1006-2483.2024.02.023.辛金换, 郭亚卿, 刘洋, 等. 肝硬化患者合并细菌感染的生存率及生活质量分析[J]. 公共卫生与预防医学, 2024, 35( 2): 101- 105. DOI: 10.3969/j.issn.1006-2483.2024.02.023. [13] YANG GM, XU LY, WANG RM, et al. Structures of the human Wilson disease copper transporter ATP7B[J]. Cell Rep, 2023, 42( 5): 112417. DOI: 10.1016/j.celrep.2023.112417. [14] WANG Y, ZHAO HJ, SHAO YZ, et al. Copper or/and arsenic induces autophagy by oxidative stress-related PI3K/AKT/mTOR pathways and cascaded mitochondrial fission in chicken skeletal muscle[J]. J Inorg Biochem, 2018, 188: 1- 8. DOI: 10.1016/j.jinorgbio.2018.08.001. [15] TSANG T, POSIMO JM, GUDIEL AA, et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma[J]. Nat Cell Biol, 2020, 22( 4): 412- 424. DOI: 10.1038/s41556-020-0481-4. [16] LI XR, BAI YM, HUO HH, et al. Long-term copper exposure induces mitochondrial dynamics disorder and mitophagy in the cerebrum of pigs[J]. Biol Trace Elem Res, 2023, 201( 3): 1197- 1204. DOI: 10.1007/s12011-022-03224-4. [17] WANG Q, ZHOU F, YUAN YC, et al. Clinical feature and gene mutation in patients with hepatolenticular degeneration: An analysis of 79 cases[J]. J Pract Hepatol, 2024, 27( 1): 60- 63. DOI: 10.3969/j.issn.1672-5069.2024.01.016.王琦, 周峰, 袁宇初, 等. 肝豆状核变性患者临床特征及基因突变位点分析[J]. 实用肝脏病杂志, 2024, 27( 1): 60- 63. DOI: 10.3969/j.issn.1672-5069.2024.01.016. [18] GARBUZ MM, OVCHINNIKOVA AA, KUMEIKO VV. Design, optimization and validation of the ARMS PCR protocol for the rapid diagnosis of Wilson’s disease using a panel of 14 common mutations for the European population[J]. Genes(Basel), 2022, 13( 11): 1940. DOI: 10.3390/genes-13111940. [19] LUCERO C, VERNA EC. The role of sarcopenia and frailty in hepatic encephalopathy management[J]. Clin Liver Dis, 2015, 19( 3): 507- 528. DOI: 10.1016/j.cld.2015.04.003. [20] DASARATHY S, MCCULLOUGH AJ, MUC S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin[J]. J Hepatol, 2011, 54( 5): 915- 921. DOI: 10.1016/j.jhep.2010.08.032. [21] OLDE DAMINK SWM, JALAN R, REDHEAD DN, et al. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS[J]. Hepatology, 2002, 36( 5): 1163- 1171. DOI: 10.1053/jhep.2002.36497. [22] LI YY, GUO YX, WANG XZ, et al. Association between sarcopenia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: A systematic review and meta-analysis[J]. Abdom Radiol(NY), 2024, 49( 2): 575- 585. DOI: 10.1007/s00261-023-04095-6. [23] OWEN OE, REICHLE FA, MOZZOLI MA, et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis[J]. J Clin Invest, 1981, 68( 1): 240- 252. DOI: 10.1172/jci110240. [24] HSU YC, HUANG DQ, NGUYEN MH. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 8): 524- 537. DOI: 10.1038/s41575-023-00760-9. [25] European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease[J]. J Hepatol, 2019, 70( 1): 172- 193. DOI: 10.1016/j.jhep.2018.06.024. [26] WANG N, LI J, LI X, et al. Risk factors for hepatic encephalopathy in patients with liver cirrhosis: A retrospective study[J]. J Third Mil Med Univ, 2019, 41( 9): 885- 890. DOI: 10.16016/j.1000-5404.201901048.王娜, 李娟, 李霞, 等. 肝硬化患者肝性脑病发生风险的回顾性研究[J]. 第三军医大学学报, 2019, 41( 9): 885- 890. DOI: 10.16016/j.1000-5404.201901048. [27] ZHAO Y, BECCE F, BALMER R, et al. Prognostic value of CT-based skeletal muscle and adipose tissue mass and quality parameters in patients with liver metastases and intrahepatic cholangiocarcinoma undergoing Yttrium-90 radioembolization[J]. Eur Radiol, 2025, 35( 3): 1415- 1427. DOI: 10.1007/s00330-025-11349-y. [28] HANAI T, SHIRAKI M, NISHIMURA K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis[J]. Nutrition, 2015, 31( 1): 193- 199. DOI: 10.1016/j.nut.2014.07.005. [29] WARNER II ER, SATAPATHY SK. Sarcopenia in the cirrhotic patient: Current knowledge and future directions[J]. J Clin Exp Hepatol, 2023, 13( 1): 162- 177. DOI: 10.1016/j.jceh.2022.06.005. [30] YOSHIJI H, NAGOSHI S, AKAHANE T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020[J]. J Gastroenterol, 2021, 56( 7): 593- 619. DOI: 10.1007/s00535-021-01788-x. [31] ZHU YJ, LI W, ZHAO SS. Research advances in the association between sarcopenia and hepatic encephalopathy in patients with liver cirrhosis[J]. J Clin Hepatol, 2023, 39( 3): 671- 676. DOI: 10.3969/j.issn.1001-5256.2023.03.030.朱伊静, 李伟, 赵守松. 肝硬化患者肌肉减少症与肝性脑病的关系[J]. 临床肝胆病杂志, 2023, 39( 3): 671- 676. DOI: 10.3969/j.issn.1001-5256.2023.03.030. [32] ZHANG L, LI H, TANG SH. Thoughts on the diagnosis and treatment of hepatic encephalopathy associated with blood ammonia in liver cirrhosis[J]. J Clin Hepatol, 2023, 39( 12): 2942- 2945. DOI: 10.3969/j.issn.1001-5256.2023.12.028.张亮, 李浩, 汤善宏. 肝硬化血氨相关肝性脑病的诊治思考[J]. 临床肝胆病杂志, 2023, 39( 12): 2942- 2945. DOI: 10.3969/j.issn.1001-5256.2023.12.028. [33] WIJARNPREECHA K, WERLANG M, PANJAWATANAN P, et al. Association between sarcopenia and hepatic encephalopathy: A systematic review and meta-analysis[J]. Ann Hepatol, 2020, 19( 3): 245- 250. DOI: 10.1016/j.aohep.2019.06.007. -



 PDF下载 ( 16470 KB)
PDF下载 ( 16470 KB)

 下载:
下载: