中药调控肿瘤相关巨噬细胞对肝细胞癌的治疗作用与机制
DOI: 10.12449/JCH250630
Therapeutic effect of traditional Chinese medicine in liver cancer by regulating tumor-associated macrophages and its mechanism
-
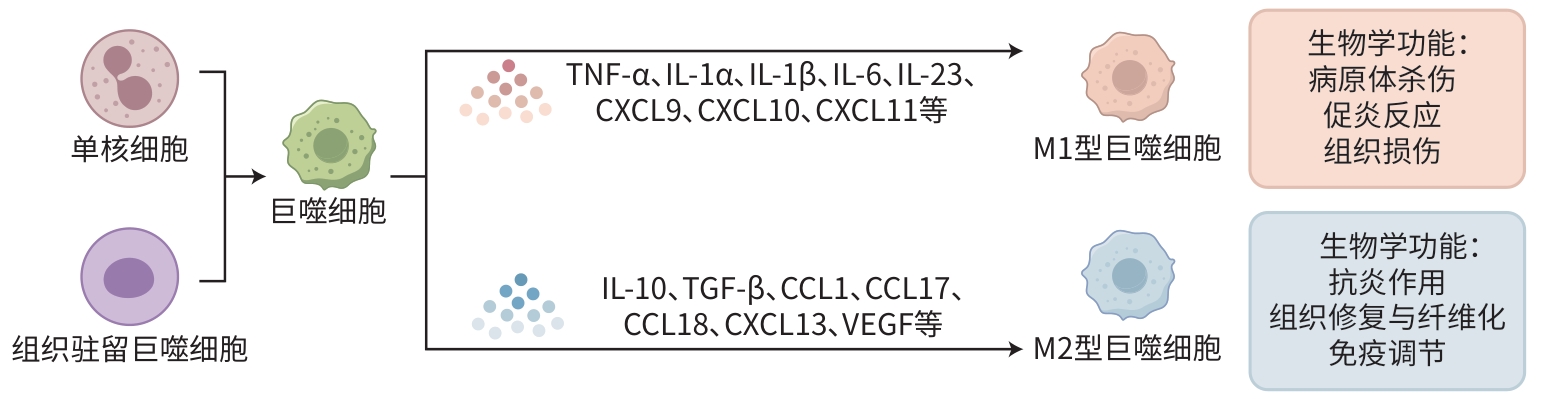
摘要: 肝细胞癌(HCC)作为全球性高发病率、高病死率的疾病,其发生发展与肿瘤微环境和肿瘤相关巨噬细胞(TAM)的相互作用密切相关。TAM在HCC免疫抑制、免疫逃逸、细胞增殖、侵袭和转移以及药物耐受性方面发挥着重要作用。中药凭借其独特的治疗理念和方法,在调控TAM、改善HCC预后方面展现出巨大潜力。本文综述了中药调控TAM在HCC治疗中的作用及其分子机制,探讨了TAM在HCC进展中的关键作用,并基于中医理论,系统分析了中药成分对TAM募集、极化、活性及相关因子表达的影响。当前研究发现,中药可通过调节TAM的极化状态,促进M1型抗肿瘤巨噬细胞的形成,抑制M2型肿瘤巨噬细胞的活性,从而在抑制HCC细胞增殖、促进凋亡、抗血管生成和增强免疫应答等方面发挥作用。此外,本文还总结了中药单体、复方及新型制剂在HCC治疗中的分子靶点和作用机制,如通过抑制TAM分泌细胞因子、调节信号通路、影响代谢途径等,为中药在HCC治疗中的应用提供了科学依据,同时为HCC的免疫治疗提供了新思路。Abstract: Liver cancer has high prevalence and mortality rates around the world, and its development and progression are closely associated with the interaction between the tumor microenvironment and tumor-associated macrophages (TAMs). TAMs play a significant role in immune suppression, immune escape, cell proliferation, invasion, metastasis, and drug resistance in liver cancer. Traditional Chinese medicine (TCM), with its unique therapeutic concepts and methods, has shown great potential in regulating TAMs and improving the prognosis of liver cancer. This article reviews the role and molecular mechanisms of TCM in regulating TAMs for the treatment of liver cancer, discusses the key role of TAMs in the progression of liver cancer, and analyzes the impact of Chinese medicinal components on the recruitment, polarization, and activity of TAMs and the expression of related factors based on TCM theory. Studies have shown that TCM can regulate the polarization state of TAMs, promote the formation of M1-type antitumor macrophages, and inhibit the activity of M2-type tumor macrophages, thereby playing a role in inhibiting the proliferation of liver cancer cells, promoting apoptosis, inhibiting angiogenesis, and enhancing immune response. In addition, this article also summarizes the molecular targets and mechanisms of action of TCM monomers, compound prescriptions, and novel preparations in the treatment of liver cancer, such as inhibiting the secretion of cytokines by TAMs, regulating signaling pathways, and affecting metabolic pathways, in order to provide a scientific basis for the application of TCM in liver cancer treatment and offer new ideas for immunotherapy for liver cancer.
-
Key words:
- Liver Neoplasms /
- Tumor-Associated Macrophages /
- Traditional Chinese Drugs
-
表 1 中药单体及其活性成分调控TAM的作用机制
Table 1. Mechanisms of action of traditional Chinese medicine monomers and their active components in regulating TAM
分类 中药活性
成分来源 实验类型 实验模型
(细胞/动物)作用机制
(TAM极化方向+信号通路)参考
文献生物碱类 澳洲茄边碱 龙葵 体内 BALB/c裸鼠 M1↓,M2↑,抑制LIF/miR-192-5p/CYR61/
Akt信号轴,诱导HCC细胞的凋亡和自噬[30] 岩黄连总
生物碱岩黄连 体内 昆明小鼠 M1↑,M2↓,抑制PI3K/Akt信号通路和
HCC细胞的生长[31] 青藤碱 青风藤 体内+体外 昆明小鼠+RAW264.7细胞+
H22细胞M1↑,M2↓,下调α7nAChR的表达,抑制
HCC细胞的生长[32] 蒽醌类 大黄素 大黄、
芦荟等体外+体内 HepG2细胞+Huh7细胞+
THP-1细胞+BALB/c裸鼠M1↑,M2↓,抑制miR-26a/TGF-β1/Akt
信号通路和HCC细胞的增殖和侵袭[34] 苯酞类 藁本内酯 川芎、当归 体外 HepG2+THP-1+HL-7702
细胞M2↓,抑制IL-6受体/STAT3信号通路和
HCC进展[35] 萜类 斑蝥素 斑蝥 体内 BALB/c裸鼠 M1↑,M2↓,通过抑制β-catenin和STAT3
信号通路来抑制肿瘤细胞生存和侵袭[36] 黄酮类 槲皮素 黄芩、
槐花等体外+体内 H22细胞+HepG2细胞+
BALB/c裸鼠M1↑,M2↓,通过调节巨噬细胞极化,协
同NF-κB通路调节自噬,抑制HCC发展[39] 黄芩苷 黄芩 体内+体外 BALB/c裸鼠+MHCC97L细胞 M1↑,M2↓,通过自噬诱导RelB/p52
信号通路的激活,抑制HCC的发展[40] 甾体类 蟾毒灵 蟾蜍 体内+体外 C57BL/6小鼠+BALB/c裸鼠+
Hepa1-6细胞M1↑,M2↓,激活NF-κB信号通路和免
疫反应,抑制HCC的发生[41] cynsaccatol L 甘遂 体外 HepG2细胞+RAW264.7细胞 M2↓,抑制Akt/ERK信号通路,诱导HCC
细胞的凋亡[42] 皂苷类 黄芪甲苷Ⅳ 黄芪 体内+体外 BALB/c裸鼠+Huh-7细胞+
THP-1细胞M1↑,M2↓,抑制TLR4/NF-κB/STAT3
信号通路和HCC细胞的生长和侵袭[43] 多糖类 灵芝多糖 灵芝 体内+体外 C57BL/6小鼠+RAW264.7
细胞M1↑,M2↓,激活MAPK/NF-κB信号通路,
抑制HCC的发展[44] 灵芝孢子粉
多糖灵芝孢子 体外 BALB/c裸鼠 M1↑,M2↓,抑制PI3K/Akt信号通路,诱
导HCC细胞的凋亡[45] 酚类 单宁 毛诃子 体内+体外 C57BL/6小鼠+BMDM细胞 M1↑,M2↓,增强CD8+ T细胞的细胞毒
性功能[46] 注:↓表示减少;↑表示增加;CYR61,富含半胱氨酸蛋白61。
表 2 中药复方及配伍药调控TAM的作用机制
Table 2. Mechanisms of action of traditional Chinese medicine formulas and compatible herb pairs in regulating TAM
复方 组成 实验类型 实验模型
(细胞/动物)作用机制
(TAM极化方向+信号通路)参考文献 参莲汤 党参、陈皮、半枝莲 体内+体外 C57BL/6小鼠+
Hepa1-6细胞M1↑,M2↓,抑制AMPK/p38信号通路,减少免
疫抑制微环境的形成[47] 西黄丸 牛黄、麝香、醋制乳香和醋
制没药体外+体内 HepG2细胞+
昆明小鼠M1↓,M2↑,促进癌细胞的凋亡;调节PD-1/PD-
L1信号通路,抑制免疫逃逸[48] 益脾养肝方 白术、党参、熟地黄、鳖甲、
枸杞子、半枝莲、郁金、姜
黄、白花蛇舌草体内 Wistar雄性大鼠 M1↓,通过调节免疫反应和抗炎作用,显示出对
HCC前病变的潜在治疗作用[49] 黄芩汤 黄芩、白芍、甘草、大枣 体内 BALB/c裸鼠 M1↑,促进肿瘤细胞凋亡,调节TME中的炎症和
自噬过程,增强巨噬细胞浸润以及激活ERK1/2
信号通路,增强索拉非尼的抗HCC作用[50] -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] ALLEMANI C, MATSUDA T, di CARLO V, et al. Global surveillance of trends in cancer survival 2000-14(CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391( 10125): 1023- 1075. DOI: 10.1016/S0140-6736(17)33326-3. [3] ZHANG CY, YANG M, ERICSSON AC. Function of macrophages in disease: Current understanding on molecular mechanisms[J]. Front Immunol, 2021, 12: 620510. DOI: 10.3389/fimmu.2021.620510. [4] ZHANG X, JI LL, LI MO. Control of tumor-associated macrophage responses by nutrient acquisition and metabolism[J]. Immunity, 2023, 56( 1): 14- 31. DOI: 10.1016/j.immuni.2022.12.003. [5] YUAN RF, LI SF, GENG H, et al. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis[J]. Int Immunopharmacol, 2017, 49: 30- 37. DOI: 10.1016/j.intimp.2017.05.014. [6] Branch of Hepatobiliary Diseases, China Association of Chinese Medicine. Guideline for traditional Chinese medicine diagnosis and treatment of primary liver cancer[J]. J Clin Hepatol, 2024, 40( 5): 919- 927. DOI: 10.12449/JCH240509.中华中医药学会肝胆病分会. 原发性HCC中医诊疗指南[J]. 临床肝胆病杂志, 2024, 40( 5): 919- 927. DOI: 10.12449/JCH240509. [7] ZHANG Y, LOU YN, WANG JB, et al. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment[J]. Front Immunol, 2021, 11: 609705. DOI: 10.3389/fimmu.2020.609705. [8] CHU XY, TIAN Y, LV C. Decoding the spatiotemporal heterogeneity of tumor-associated macrophages[J]. Mol Cancer, 2024, 23( 1): 150. DOI: 10.1186/s12943-024-02064-1. [9] DANIEL B, NAGY G, HORVATH A, et al. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages[J]. Nucleic Acids Res, 2018, 46( 9): 4425- 4439. DOI: 10.1093/nar/gky157. [10] LIU HX, AMAKYE WK, REN JY. Codonopsis pilosula polysaccharide in synergy with dacarbazine inhibits mouse melanoma by repolarizing M2-like tumor-associated macrophages into M1-like tumor-associated macrophages[J]. Biomed Pharmacother, 2021, 142: 112016. DOI: 10.1016/j.biopha.2021.112016. [11] VERGADI E, IERONYMAKI E, LYRONI K, et al. Akt signaling pathway in macrophage activation and M1/M2 polarization[J]. J Immunol, 2017, 198( 3): 1006- 1014. DOI: 10.4049/jimmunol.1601515. [12] KONG LX, ZHOU YJ, BU H, et al. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice[J]. J Exp Clin Cancer Res, 2016, 35( 1): 131. DOI: 10.1186/s13046-016-0412-1. [13] YAO CY, WU SL, KONG J, et al. Angiogenesis in hepatocellular carcinoma: Mechanisms and anti-angiogenic therapies[J]. Cancer Biol Med, 2023, 20( 1): 25- 43. DOI: 10.20892/j.issn.2095-3941.2022.0449. [14] JAKAB M, ROSTALSKI T, LEE KH, et al. TIE2 receptor in tumor-infiltrating macrophages is dispensable for tumor angiogenesis and tumor relapse after chemotherapy[J]. Cancer Res, 2022, 82( 7): 1353- 1364. DOI: 10.1158/0008-5472.CAN-21-3181. [15] ZANG MY, LI Y, HE H, et al. IL-23 production of liver inflammatory macrophages to damaged hepatocytes promotes hepatocellular carcinoma development after chronic hepatitis B virus infection[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864( 12): 3759- 3770. DOI: 10.1016/j.bbadis.2018.10.004. [16] GRAHAM N, POLLARD JW. An acid trip activates protumoral macrophages to promote hepatocellular carcinoma malignancy[J]. J Clin Invest, 2022, 132( 7): e158562. DOI: 10.1172/JCI158562. [17] LI MJ, HE LY, ZHU J, et al. Targeting tumor-associated macrophages for cancer treatment[J]. Cell Biosci, 2022, 12( 1): 85. DOI: 10.1186/s13578-022-00823-5. [18] WU QC, ZHOU WH, YIN SY, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer[J]. Hepatology, 2019, 70( 1): 198- 214. DOI: 10.1002/hep.30593. [19] YIN Y, FENG WB, CHEN J, et al. Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: From bench to bedside[J]. Exp Hematol Oncol, 2024, 13( 1): 72. DOI: 10.1186/s40164-024-00539-x. [20] CHEN YX, WEN HH, ZHOU C, et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells[J]. Exp Cell Res, 2019, 378( 1): 41- 50. DOI: 10.1016/j.yexcr.2019.03.005. [21] YAN WJ, LIU X, MA HX, et al. TIM-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages[J]. Gut, 2015, 64( 10): 1593- 1604. DOI: 10.1136/gutjnl-2014-307671. [22] JIANG J, WANG GZ, WANG Y, et al. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6[J]. Exp Cell Res, 2018, 367( 1): 81- 88. DOI: 10.1016/j.yexcr.2018.03.025. [23] DONG NN, SHI XY, WANG SH, et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma[J]. Br J Cancer, 2019, 121( 1): 22- 33. DOI: 10.1038/s41416-019-0482-x. [24] WANG HC, HAUNG LY, WANG CJ, et al. Tumor-associated macrophages promote resistance of hepatocellular carcinoma cells against sorafenib by activating CXCR2 signaling[J]. J Biomed Sci, 2022, 29( 1): 99. DOI: 10.1186/s12929-022-00881-4. [25] FU XT, SONG K, ZHOU J, et al. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma[J]. Cancer Cell Int, 2019, 19: 71. DOI: 10.1186/s12935-019-0771-8. [26] WANG YH, WANG ZJ, JIA F, et al. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment[J]. Bioact Mater, 2022, 17: 147- 161. DOI: 10.1016/j.bioactmat.2022.01.003. [27] NIU ZS, WANG WH, NIU XJ. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma[J]. World J Gastroenterol, 2022, 28( 46): 6433- 6477. DOI: 10.3748/wjg.v28.i46.6433. [28] CHEN JH, LIN ZF, LIU L, et al. GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages[J]. Signal Transduct Target Ther, 2021, 6( 1): 397. DOI: 10.1038/s41392-021-00784-0. [29] NING WR, JIANG D, LIU XC, et al. Carbonic anhydrase XII mediates the survival and prometastatic functions of macrophages in human hepatocellular carcinoma[J]. J Clin Invest, 2022, 132( 7): e153110. DOI: 10.1172/JCI153110. [30] YIN SS, JIN WK, QIU YL, et al. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment[J]. J Hematol Oncol, 2022, 15( 1): 32. DOI: 10.1186/s13045-022-01248-w. [31] SU Q, CHEN HR, LU J, et al. Experimental study of corydalis saxicola total alkaloids inhibiting M2-type macrophages against mice liver cancer[J]. J Chin Med Materials, 2023, 46( 7): 1760- 1765. DOI: 10.13863/j.issn1001-4454.2023.07.031.苏倩, 陈好然, 陆洁, 等. 岩黄连总生物碱抑制M2型巨噬细胞抗小鼠HCC实验研究[J]. 中药材, 2023, 46( 7): 1760- 1765. DOI: 10.13863/j.issn1001-4454.2023.07.031. [32] LIU XY, CAO MD, LAN Y, et al. Effects of sinomenine on α7nAChR-involved M2 polarization of macrophages and TAM polarization in hepatoma ascitic tumor mouse model[J]. Tradit Chin Drug Res Clin Pharmacol, 2022, 33( 12): 1645- 1653. DOI: 10.19378/j.issn.1003-9783.2022.12.008.刘新迎, 曹敏蝶, 蓝燕, 等. 青藤碱对α7nAChR参与的巨噬细胞M2极化和小鼠HCCTAM极化的干预作用[J]. 中药新药与临床药理, 2022, 33( 12): 1645- 1653. DOI: 10.19378/j.issn.1003-9783.2022.12.008. [33] IWANOWYCZ S, WANG JF, ALTOMARE D, et al. Emodin bidirectionally modulates macrophage polarization and epigenetically regulates macrophage memory[J]. J Biol Chem, 2016, 291( 22): 11491- 11503. DOI: 10.1074/jbc.M115.702092. [34] RAHMAN MA, RAKIB-UZ-ZAMAN SM, CHAKRABORTI S, et al. Advancements in utilizing natural compounds for modulating autophagy in liver cancer: Molecular mechanisms and therapeutic targets[J]. Cells, 2024, 13( 14): 1186. DOI: 10.3390/cells13141186. [35] YANG JK, XING ZY. Ligustilide counteracts carcinogenesis and hepatocellular carcinoma cell-evoked macrophage M2 polarization by regulating yes-associated protein-mediated interleukin-6 secretion[J]. Exp Biol Med(Maywood), 2021, 246( 17): 1928- 1937. DOI: 10.1177/15353702211010420. [36] LU S, GAO Y, HUANG XL, et al. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization[J]. Int J Biol Sci, 2014, 10( 4): 415- 425. DOI: 10.7150/ijbs.8002. [37] JIANG T, HUANG JB, XU CY, et al. Arsenic trioxide cooperate cryptotanshinone exerts antitumor effect by medicating macrophage polarization through glycolysis[J]. J Immunol Res, 2022, 2022: 2619781. DOI: 10.1155/2022/2619781. [38] JI Y, LI L, MA YX, et al. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice[J]. J Nutr Biochem, 2019, 69: 108- 119. DOI: 10.1016/j.jnutbio.2019.03.018. [39] WU RX, ZHOU T, XIONG JQ, et al. Quercetin, the ingredient of Xihuang pills, inhibits hepatocellular carcinoma by regulating autophagy and macrophage polarization[J]. Front Biosci(Landmark Ed), 2022, 27( 12): 323. DOI: 10.31083/j.fbl2712323. [40] TAN HY, WANG N, MAN K, et al. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin[J]. Cell Death Dis, 2015, 6( 10): e1942. DOI: 10.1038/cddis.2015.271. [41] YU Z, LI YY, LI Y, et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma[J]. J Immunother Cancer, 2022, 10( 5): e004297. DOI: 10.1136/jitc-2021-004297. [42] FENG XY, LI JC, LI HM, et al. Bioactive C21 steroidal glycosides from Euphorbia kansui promoted HepG2 cell apoptosis via the degradation of ATP1A1 and inhibited macrophage polarization under co-cultivation[J]. Molecules, 2023, 28( 6): 2830. DOI: 10.3390/molecules28062830. [43] CHEN YQ, FAN WS, ZHAO YY, et al. Progress in the regulation of immune cells in the tumor microenvironment by bioactive compounds of traditional Chinese medicine[J]. Molecules, 2024, 29( 10): 2374. DOI: 10.3390/molecules29102374. [44] LI GL, TANG JF, TAN WL, et al. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway[J]. Food Funct, 2023, 14( 7): 3155- 3168. DOI: 10.1039/d2fo02191a. [45] SONG M, LI ZH, GU HS, et al. Ganoderma lucidum spore polysaccharide inhibits the growth of hepatocellular carcinoma cells by altering macrophage polarity and induction of apoptosis[J]. J Immunol Res, 2021, 2021: 6696606. DOI: 10.1155/2021/6696606. [46] CHANG ZH, ZHANG QN, HU Q, et al. Tannins in Terminalia bellirica inhibits hepatocellular carcinoma growth via re-educating tumor-associated macrophages and restoring CD8+T cell function[J]. Biomed Pharmacother, 2022, 154: 113543. DOI: 10.1016/j.biopha.2022.113543. [47] LI WX, YOU LP, LIN JC, et al. An herbal formula Shenlian decoction upregulates M1/M2 macrophage proportion in hepatocellular carcinoma by suppressing complement cascade[J]. Biomed Pharmacother, 2024, 177: 116943. DOI: 10.1016/j.biopha.2024.116943. [48] WANG Y, WANG WH, LIU KL, et al. The mechanism of Xihuang pills’ intervention in the tumour immune microenvironment for the treatment of liver cancer based on the STAT3-PDL1 pathway[J]. J Ethnopharmacol, 2024, 331: 118278. DOI: 10.1016/j.jep.2024.118278. [49] HUANG Y, GOU XY, GUAN X, et al. Exploration on the mechanism of Yipi Yanggan prescription for the treatment of liver precancerous lesion based on M1 type macrophage polarization-chronic inflammation-liver cell malignant transformation[J]. Chin J Inf Tradit Chin Med, 2024, 31( 10): 81- 88. DOI: 10.19879/j.cnki.1005-5304.202404384.黄玉, 苟雪源, 关茜, 等. 基于M1型巨噬细胞极化-慢性炎症-肝细胞恶变探究益脾养肝方治疗HCC前病变作用机制[J]. 中国中医药信息杂志, 2024, 31( 10): 81- 88. DOI: 10.19879/j.cnki.1005-5304.202404384. [50] LAM W, JIANG ZL, GUAN FL, et al. PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment[J]. Sci Rep, 2015, 5: 9384. DOI: 10.1038/srep09384. [51] WANG X, TAN Y, ZHANG YL, et al. The novel glycyrrhetinic acid-tetramethylpyrazine conjugate TOGA induces anti-hepatocarcinogenesis by inhibiting the effects of tumor-associated macrophages on tumor cells[J]. Pharmacol Res, 2020, 161: 105233. DOI: 10.1016/j.phrs.2020.105233. [52] HUANG LQ, SHI XM, WANG JR, et al. Preparation and polarization activity research of Astragalus polysaccharide-superparamagnetic iron oxide nanocomposite[J]. Acta Pharm Sin, 2023, 58( 3): 779- 788. DOI: 10.16438/j.0513-4870.2022-1059.黄琳清, 史新萌, 王静蓉, 等. 黄芪多糖-超顺磁性氧化铁纳米复合物的制备及其诱导巨噬细胞极化的活性研究[J]. 药学学报, 2023, 58( 3): 779- 788. DOI: 10.16438/j.0513-4870.2022-1059. [53] XU ZY, HUANG Y, WU YH, et al. Glycyrrhizic acid-lipid framework nanovehicle loading triptolide for combined immunochemotherapy[J]. ACS Appl Mater Interfaces, 2023, 15( 35): 41337- 41350. DOI: 10.1021/acsami.3c08003. -



 PDF下载 ( 977 KB)
PDF下载 ( 977 KB)

 下载:
下载:


