液体活检在肝细胞癌诊断及治疗中的应用
DOI: 10.12449/JCH250629
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:李玉龙负责资料分析,撰写论文和最终定稿;张欣欣负责课题设计,拟定写作思路,指导修改论文。
Research advances in the application of liquid biopsy in the diagnosis and treatment of hepatocellular carcinoma
-
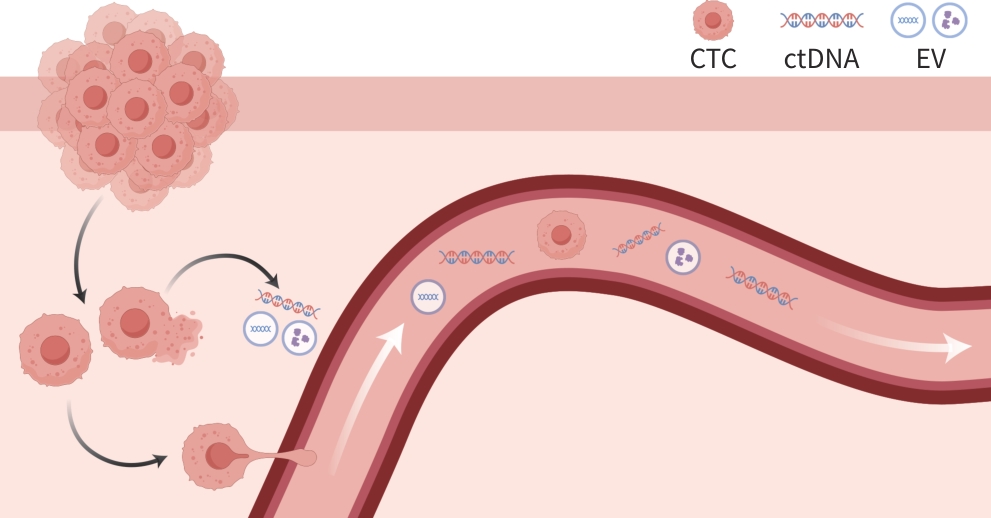
摘要: 肝细胞癌早期无明显特异性症状,确诊往往已是疾病晚期,预后较差。如能在早期及时确诊并采取有效治疗可以显著延长患者生存期。液体活检技术通过检测分析肿瘤相关生物标志物,包括循环肿瘤细胞、循环肿瘤DNA和细胞外囊泡等,可无创获得肿瘤相关信息,用于疾病的早期诊断、分子病理学分型及预后预测等。本文概述液体活检技术在肝细胞癌诊断及治疗中的临床应用研究进展。Abstract: Hepatocellular carcinoma is one of the most common cancers, and due to the lack of obvious specific symptoms in its early stage, patients are often in the advanced stage at the time of diagnosis and tend to have a poor prognosis. Timely diagnosis and effective treatment in the early stage can help to prolong the survival time of patients. Liquid biopsy is a noninvasive technique that can obtain the information of tumor by detecting and analyzing related biomarkers, including circulating tumor cells, circulating tumor DNA, and extracellular vesicles, thereby contributing to early diagnosis, molecular pathological typing, and prognosis prediction. This article reviews the research advances in the application of liquid biopsy in the diagnosis and treatment of hepatocellular carcinoma.
-
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [2] MCGLYNN KA, PETRICK JL, EL-SERAG HB. Epidemiology of hepatocellular carcinoma[J]. Hepatology, 2021, 73( Suppl 1): 4- 13. DOI: 10.1002/hep.31288. [3] KULIK L, EL-SERAG HB. Epidemiology and management of hepatocellular carcinoma[J]. Gastroenterology, 2019, 156( 2): 477- 491. e 1. DOI: 10.1053/j.gastro.2018.08.065. [4] CHIDAMBARANATHAN-REGHUPATY S, FISHER PB, SARKAR D. Hepatocellular carcinoma(HCC): Epidemiology, etiology and molecular classification[J]. Adv Cancer Res, 2021, 149: 1- 61. DOI: 10.1016/bs.acr.2020.10.001. [5] AHN JC, TENG PC, CHEN PJ, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma[J]. Hepatology, 2021, 73( 1): 422- 436. DOI: 10.1002/hep.31165. [6] van de STOLPE A, PANTEL K, SLEIJFER S, et al. Circulating tumor cell isolation and diagnostics: Toward routine clinical use[J]. Cancer Res, 2011, 71( 18): 5955- 5960. DOI: 10.1158/0008-5472.CAN-11-1254. [7] LOW WS, WAN ABAS WA. Benchtop technologies for circulating tumor cells separation based on biophysical properties[J]. Biomed Res Int, 2015, 2015: 239362. DOI: 10.1155/2015/239362. [8] de BOER CJ, van KRIEKEN JH, JANSSEN-VAN RHIJN CM, et al. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver[J]. J Pathol, 1999, 188( 2): 201- 206. DOI: 3.0.CO;2-8">10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [9] HUANG XY, LI F, LI TT, et al. A clinically feasible circulating tumor cell sorting system for monitoring the progression of advanced hepatocellular carcinoma[J]. J Nanobiotechnology, 2023, 21( 1): 25. DOI: 10.1186/s12951-023-01783-9. [10] LI W, LIU JB, HOU LK, et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring[J]. Mol Cancer, 2022, 21( 1): 25. DOI: 10.1186/s12943-022-01505-z. [11] van der VAART M, PRETORIUS PJ. The origin of circulating free DNA[J]. Clin Chem, 2007, 53( 12): 2215. DOI: 10.1373/clinchem.2007.092734. [12] DIEHL F, SCHMIDT K, CHOTI MA, et al. Circulating mutant DNA to assess tumor dynamics[J]. Nat Med, 2008, 14( 9): 985- 990. DOI: 10.1038/nm.1789. [13] MARTIN-ALONSO C, TABRIZI S, XIONG K, et al. Priming agents transiently reduce the clearance of cell-free DNA to improve liquid biopsies[J]. Science, 2024, 383( 6680): eadf2341. DOI: 10.1126/science.adf2341. [14] NIKANJAM M, KATO S, KURZROCK R. Liquid biopsy: Current technology and clinical applications[J]. J Hematol Oncol, 2022, 15( 1): 131. DOI: 10.1186/s13045-022-01351-y. [15] YE QW, LING SB, ZHENG SS, et al. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA[J]. Mol Cancer, 2019, 18( 1): 114. DOI: 10.1186/s12943-019-1043-x. [16] GUO SY, HUANG J, LI GP, et al. The role of extracellular vesicles in circulating tumor cell-mediated distant metastasis[J]. Mol Cancer, 2023, 22( 1): 193. DOI: 10.1186/s12943-023-01909-5. [17] von FELDEN J, GARCIA-LEZANA T, DOGRA N, et al. Unannotated small RNA clusters associated with circulating extracellular vesicles detect early stage liver cancer[J]. Gut, 2021. DOI: 10.1136/gutjnl-2021-325036.[ Online ahead of print] [18] LEE YT, TRAN BV, WANG JJ, et al. The role of extracellular vesicles in disease progression and detection of hepatocellular carcinoma[J]. Cancers(Basel), 2021, 13( 12): 3076. DOI: 10.3390/cancers13123076. [19] YU D, LI YX, WANG MY, et al. Exosomes as a new frontier of cancer liquid biopsy[J]. Mol Cancer, 2022, 21( 1): 56. DOI: 10.1186/s12943-022-01509-9. [20] ZHU ZQ, HU EY, SHEN H, et al. The functional and clinical roles of liquid biopsy in patient-derived models[J]. J Hematol Oncol, 2023, 16( 1): 36. DOI: 10.1186/s13045-023-01433-5. [21] YU JJ, XIAO W, DONG SL, et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma[J]. BMC Cancer, 2018, 18( 1): 835. DOI: 10.1186/s12885-018-4744-4. [22] KELLEY RK, MAGBANUA MJM, BUTLER TM, et al. Circulating tumor cells in hepatocellular carcinoma: A pilot study of detection, enumeration, and next-generation sequencing in cases and controls[J]. BMC Cancer, 2015, 15: 206. DOI: 10.1186/s12885-015-1195-z. [23] ZHAO LN, SONG JG, SUN YL, et al. Tumor-derived proliferative CTCs and CTC clusters predict aggressiveness and early recurrence in hepatocellular carcinoma patients[J]. Cancer Med, 2023, 12( 13): 13912- 13927. DOI: 10.1002/cam4.5946. [24] YEO W, WONG N, WONG WL, et al. High frequency of promoter hypermethylation of RASSF1A in tumor and plasma of patients with hepatocellular carcinoma[J]. Liver Int, 2005, 25( 2): 266- 272. DOI: 10.1111/j.1478-3231.2005.01084.x. [25] IIZUKA N, SAKAIDA I, MORIBE T, et al. Elevated levels of circulating cell-free DNA in the blood of patients with hepatitis C virus-associated hepatocellular carcinoma[J]. Anticancer Res, 2006, 26( 6C): 4713- 4719. [26] CHEN K, ZHANG H, ZHANG LN, et al. Value of circulating cell-free DNA in diagnosis of hepatocelluar carcinoma[J]. World J Gastroenterol, 2013, 19( 20): 3143- 3149. DOI: 10.3748/wjg.v19.i20.3143. [27] ONO A, FUJIMOTO A, YAMAMOTO Y, et al. Circulating tumor DNA analysis for liver cancers and its usefulness as a liquid biopsy[J]. Cell Mol Gastroenterol Hepatol, 2015, 1( 5): 516- 534. DOI: 10.1016/j.jcmgh.2015.06.009. [28] ZHU GQ, LIU WR, TANG Z, et al. Serial circulating tumor DNA to predict early recurrence in patients with hepatocellular carcinoma: A prospective study[J]. Mol Oncol, 2022, 16( 2): 549- 561. DOI: 10.1002/1878-0261.13105. [29] LIAO WJ, YANG HY, XU HF, et al. Noninvasive detection of tumor-associated mutations from circulating cell-free DNA in hepatocellular carcinoma patients by targeted deep sequencing[J]. Oncotarget, 2016, 7( 26): 40481- 40490. DOI: 10.18632/oncotarget.9629. [30] SUN N, ZHANG C, LEE YT, et al. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma[J]. Hepatology, 2023, 77( 3): 774- 788. DOI: 10.1002/hep.32692. [31] GOUDA MA, JANKU F, WAHIDA A, et al. Liquid biopsy response evaluation criteria in solid tumors(LB-RECIST)[J]. Ann Oncol, 2024, 35( 3): 267- 275. DOI: 10.1016/j.annonc.2023.12.007. [32] LI J, SHI LH, ZHANG XF, et al. pERK/pAkt phenotyping in circulating tumor cells as a biomarker for sorafenib efficacy in patients with advanced hepatocellular carcinoma[J]. Oncotarget, 2016, 7( 3): 2646- 2659. DOI: 10.18632/oncotarget.6104. -



 PDF下载 ( 867 KB)
PDF下载 ( 867 KB)

 下载:
下载:


