脂肪量和肥胖相关基因在代谢相关脂肪性肝病发生发展中的作用机制及相关靶向治疗
DOI: 10.12449/JCH250625
Mechanism of action of the fat mass and obesity-associated gene in the development and progression of metabolic dysfunction-associated fatty liver disease and related targeted therapies
-
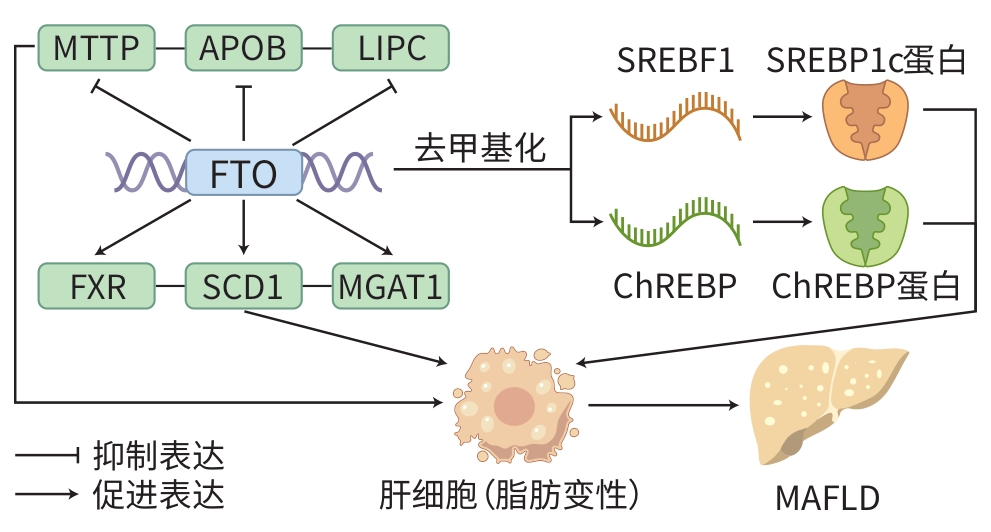
摘要: 代谢相关(非酒精性)脂肪性肝病(MAFLD)是一种常见的慢性肝脏疾病,其病理特征为肝脏内脂质堆积,且与肝脏代谢紊乱密切相关。最新研究表明,MAFLD的发生机制与特定基因的异常表达有关,特别是脂肪量和肥胖相关基因(FTO)。FTO基因表达的异常升高可能导致肝脂质代谢失衡,表现为脂肪酸合成增加和氧化减少,从而促进肝脂肪沉积和炎症反应。因此,调控FTO基因的表达或活性被认为是治疗MAFLD的潜在策略之一。目前,针对FTO基因功能的药物研究初见成效,可通过抑制FTO基因的活性,调节肝脂质代谢,并减轻肝脏的炎症损伤。本文综述了FTO基因在MAFLD发生发展中的作用机制,并总结了近年来围绕FTO基因及其相关代谢通路的药物研究进展,并展望其在该领域研究和治疗中的应用前景。
-
关键词:
- α酮戊二酸依赖性双加氧酶FTO /
- 非酒精性脂肪性肝病 /
- 脂代谢障碍 /
- 基因治疗
Abstract: Metabolic dysfunction-associated fatty liver disease (MAFLD) is a common chronic liver disease with the pathological feature of lipid accumulation in the liver, and it is closely associated with liver metabolic disorders. The latest research has shown that the pathogenesis of MAFLD is associated with the abnormal expression of specific genes, especially the fat mass and obesity-associated (FTO) gene. The abnormal activity of the FTO gene may lead to an imbalance in liver lipid metabolism, which manifests as the increase in fatty acid synthesis and the reduction in fatty acid oxidation, thereby promoting liver fat deposition and inflammatory response. Therefore, regulating the expression or activity of the FTO gene is considered one of the potential strategies for the treatment of MAFLD. At present, drug research targeting the function of the FTO gene has achieved preliminary results, and inhibition of the activity of the FTO gene can help to regulate liver lipid metabolism and alleviate liver inflammatory injury. This article reviews the mechanism of action of the FTO gene in the development and progression of MAFLD, summarizes the advances in drug research on the FTO gene and related metabolic pathways in recent years, and analyzes their application prospect in research and treatment. -
表 1 FTO基因治疗MAFLD相关药物/分子
Table 1. Related drugs/molecules for FTO treatment of MAFLD
分类 药物/分子 作用机制 应用潜力 参考文献 天然类 熊果苷 抑制FTO基因去甲基化酶活性,增加m6A
甲基化水平,调节脂质代谢相关基因表达,
减少肝脂肪积累具有天然来源和低毒性,适用于长期
治疗的MAFLD药物[44-46] TMG 增加m6A甲基化水平,减少脂肪堆积,调节
脂质代谢预防脂质代谢紊乱,治疗MAFLD [50-51] 非天然类
(药物类)EXN 激活GLP-1受体,启动PI3K/AKT信号通路,
抑制FTO基因表达,减少脂肪堆积和炎症
反应作为糖尿病和MAFLD的双效药物,改
善肝脂肪堆积,减轻炎症[41-43] COMT-Is 通过抑制FTO基因去甲基化酶活性,增加
m6A甲基化水平,调节脂质代谢相关基因表
达,减少脂肪积累显著改善MAFLD,潜在治疗其他脂质
代谢相关疾病的药物[38,47-48] DCS 调节FTO基因表达,影响m6A水平和肝脂
质代谢治疗MAFLD,具有潜在应用价值 [50-51] 非天然类
(非编码RNA
及其载体)miRNA 通过抑制FTO基因表达,调节脂质代谢,如
miR-627-5p、miR-143、miR-30b个性化治疗的潜力,可能改善MAFLD
和其他代谢性疾病[53-54] 外泌体 通过靶向FTO基因,调控葡萄糖和脂质代
谢,改善MAFLD作为MAFLD治疗的创新方法,可能为
新型治疗提供思路[52] -
[1] STEFAN N, SCHICK F, BIRKENFELD AL, et al. The role of hepatokines in NAFLD[J]. Cell Metab, 2023, 35( 2): 236- 252. DOI: 10.1016/j.cmet.2023.01.006. [2] MONSERRAT-MESQUIDA M, QUETGLAS-LLABRÉS M, BOUZAS C, et al. A greater improvement of intrahepatic fat contents after 6 months of lifestyle intervention is related to a better oxidative stress and inflammatory status in non-alcoholic fatty liver disease[J]. Antioxidants(Basel), 2022, 11( 7): 1266. DOI: 10.3390/antiox11071266. [3] COTTER TG, RINELLA M. Nonalcoholic fatty liver disease 2020: The state of the disease[J]. Gastroenterology, 2020, 158( 7): 1851- 1864. DOI: 10.1053/j.gastro.2020.01.052. [4] WU YK, ZHENG Q, ZOU BY, et al. The epidemiology of NAFLD in the Chinese mainland with analysis by adjusted gross regional domestic product: A meta-analysis[J]. Hepatol Int, 2020, 14( 2): 259- 269. DOI: 10.1007/s12072-020-10023-3. [5] HUANG YZ, CHEN H, CHEN JL, et al. Yellow tea polysaccharides protect against non-alcoholic fatty liver disease via regulation of gut microbiota and bile acid metabolism in mice[J]. Phytomedicine, 2024, 133: 155919. DOI: 10.1016/j.phymed.2024.155919. [6] JIANG HT, ZHU H, HUO GM, et al. Oudemansiella raphanipies polysaccharides improve lipid metabolism disorders in murine high-fat diet-induced non-alcoholic fatty liver disease[J]. Nutrients, 2022, 14( 19): 4092. DOI: 10.3390/nu14194092. [7] LAN N, LU Y, ZHANG YG, et al. FTO-A common genetic basis for obesity and cancer[J]. Front Genet, 2020, 11: 559138. DOI: 10.3389/fgene.2020.559138. [8] TAN J, WANG YF, DAI ZH, et al. Roles of RNA m6A modification in nonalcoholic fatty liver disease[J]. Hepatol Commun, 2023, 7( 2): e0046. DOI: 10.1097/HC9.0000000000000046. [9] WEI XH, ZHANG JL, TANG M, et al. Fat mass and obesity-associated protein promotes liver steatosis by targeting PPARα[J]. Lipids Health Dis, 2022, 21: 29. DOI: 10.1186/s12944-022-01640-y. [10] GAN XJ, DAI ZH, GE CM, et al. FTO promotes liver inflammation by suppressing m6A mRNA methylation of IL-17RA[J]. Front Oncol, 2022, 12: 989353. DOI: 10.3389/fonc.2022.989353. [11] MITTENBÜHLER MJ, SAEDLER K, NOLTE H, et al. Hepatic FTO is dispensable for the regulation of metabolism but counteracts HCC development in vivo[J]. Mol Metab, 2020, 42: 101085. DOI: 10.1016/j.molmet.2020.101085. [12] QIAN XF, ZENG P, LIU JF, et al. Research progress of enzymes related to m6A RNA methylation modification[J]. Chin J Immunol, 2023, 39( 5): 1073- 1084. DOI: 10.3969/j.issn.1000-484X.2023.05.033.钱晓芬, 曾平, 刘金富, 等. m6A RNA甲基化修饰相关酶的研究进展[J]. 中国免疫学杂志, 2023, 39( 5): 1073- 1084. DOI: 10.3969/j.issn.1000-484X.2023.05.033. [13] LI YC, SU R, DENG XL, et al. FTO in cancer: Functions, molecular mechanisms, and therapeutic implications[J]. Trends Cancer, 2022, 8( 7): 598- 614. DOI: 10.1016/j.trecan.2022.02.010. [14] XU ZY, JING X, XIONG XD. Emerging role and mechanism of the FTO gene in cardiovascular diseases[J]. Biomolecules, 2023, 13( 5): 850. DOI: 10.3390/biom13050850. [15] WU RF, CHEN YS, LIU YH, et al. m6A methylation promotes white-to-beige fat transition by facilitating Hif1a translation[J]. EMBO Rep, 2021, 22( 11): e52348. DOI: 10.15252/embr.202052348. [16] HE Y, YANG WH, GAN LL, et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol, 2021, 44( 5): 355- 365. DOI: 10.1016/j.gastrohep.2020.09.014. [17] BEN-HAIM MS, PINTO Y, MOSHITCH-MOSHKOVITZ S, et al. Dynamic regulation of N6, 2'-O-dimethyladenosine(m6Am) in obesity[J]. Nat Commun, 2021, 12( 1): 7185. DOI: 10.1038/s41467-021-27421-2. [18] YANG Z, YU GL, ZHU X, et al. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: Implications in lipid metabolic disorders[J]. Genes Dis, 2021, 9( 1): 51- 61. DOI: 10.1016/j.gendis.2021.01.005. [19] LI Y, YANG F, GAO M, et al. miR-149-3p Regulates the switch between adipogenic and osteogenic differentiation of BMSCs by targeting FTO[J]. Mol Ther Nucleic Acids, 2019, 17: 590- 600. DOI: 10.1016/j.omtn.2019.06.023. [20] CHURCH C, MOIR L, MCMURRAY F, et al. Overexpression of FTO leads to increased food intake and results in obesity[J]. Nat Genet, 2010, 42( 12): 1086- 1092. DOI: 10.1038/ng.713. [21] LI XC, JIN F, WANG BY, et al. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA[J]. Theranostics, 2019, 9( 13): 3853- 3865. DOI: 10.7150/thno.31868. [22] AĞAGÜNDÜZ D, GEZMEN-KARADAĞ M. Association of FTO common variant(rs9939609) with body fat in Turkish individuals[J]. Lipids Health Dis, 2019, 18( 1): 212. DOI: 10.1186/s12944-019-1160-y. [23] SUN DL, ZHAO TH, ZHANG Q, et al. Fat mass and obesity-associated protein regulates lipogenesis via m6A modification in fatty acid synthase mRNA[J]. Cell Biol Int, 2021, 45( 2): 334- 344. DOI: 10.1002/cbin.11490. [24] ZHAO LC, FAN TT, HAN YL, et al. Demethylase FTO activity analysis based on methyl sensitive enzyme MazF and hybridization chain reaction[J]. Sens Actuat B Chem, 2021, 341: 129983. DOI: 10.1016/j.snb.2021.129983. [25] TANG ZL, SUN C, YAN Y, et al. Aberrant elevation of FTO levels promotes liver steatosis by decreasing the m6A methylation and increasing the stability of SREBF1 and ChREBP mRNAs[J]. J Mol Cell Biol, 2023, 14( 9): mjac061. DOI: 10.1093/jmcb/mjac061. [26] ZHANG VX, CHEN A, ZHANG QY, et al. FRI-473 The oncogenic m6A demethylase FTO promotes tumorigenesis and immune escape by upregulating GPNMB in hepatocellular carcinoma[J]. J Hepatol, 2024, 80: S419- S420. DOI: 10.1016/S0168-8278(24)01335-7. [27] LI R, YAN XJ, XIAO CC, et al. FTO deficiency in older livers exacerbates ferroptosis during ischaemia/reperfusion injury by upregulating ACSL4 and TFRC[J]. Nat Commun, 2024, 15( 1): 4760. DOI: 10.1038/s41467-024-49202-3. [28] BIAN XY, SHI DM, XING KL, et al. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation[J]. Clin Transl Med, 2021, 11( 3): e352. DOI: 10.1002/ctm2.352. [29] ZHENG JH, WANG FJ, GUO HW, et al. Gut microbiota modulates differential lipid metabolism outcomes associated with FTO gene polymorphisms in response to personalized nutrition intervention[J]. Front Nutr, 2022, 9: 985723. DOI: 10.3389/fnut.2022.985723. [30] CHEN XF, GAO Y, YANG XB, et al. Relationship of FTO gene variations with NAFLD risk in Chinese men[J]. Open Life Sci, 2020, 15( 1): 860- 867. DOI: 10.1515/biol-2020-0081. [31] GU Z, BI Y, YUAN F, et al. FTO polymorphisms are associated with metabolic dysfunction-associated fatty liver disease(MAFLD) susceptibility in the older Chinese Han population[J]. Clin Interv Aging, 2020, 15: 1333- 1341. DOI: 10.2147/CIA.S254740. [32] PANKOVA ED, CHULKOV VS, GAVRILOVA ES, et al. Sequence gene variants in PPARGC1A rs8192678, PPARG2 rs1801282, FTO rs9939609, LEP rs7799039, LEPR rs1137101 and nonalcoholic fatty liver disease[J]. Saratov J Med Sci Res, 2023, 19( 3): 256- 260. DOI: 10.15275/ssmj1903256. [33] KANG HF, ZHANG ZW, YU L, et al. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation[J]. J Cell Biochem, 2018, 119( 7): 5676- 5685. DOI: 10.1002/jcb.26746. [34] CHANDRASEKARAN P, WEISKIRCHEN R. The role of SCAP/SREBP as central regulators of lipid metabolism in hepatic steatosis[J]. Int J Mol Sci, 2024, 25( 2): 1109. DOI: 10.3390/ijms25021109. [35] IIZUKA K, KEN TK, YABE D. ChREBP-mediated regulation of lipid metabolism: Involvement of the gut microbiota, liver, and adipose tissue[J]. Front Endocrinol(Lausanne), 2020, 11: 587189. DOI: 10.3389/fendo.2020.587189. [36] DO MH, OH MJ, LEE HB, et al. Bifidobacterium animalis ssp. lactis MG741 reduces body weight and ameliorates nonalcoholic fatty liver disease via improving the gut permeability and amelioration of inflammatory cytokines[J]. Nutrients, 2022, 14( 9): 1965. DOI: 10.3390/nu14091965. [37] MANZANO M, GIRON MD, SALTO R, et al. Quality more than quantity: The use of carbohydrates in high-fat diets to tackle obesity in growing rats[J]. Front Nutr, 2022, 9: 809865. DOI: 10.3389/fnut.2022.809865. [38] REN Y, HUANG P, ZHANG L, et al. Dual regulation mechanism of obesity: DNA methylation and intestinal flora[J]. Biomedicines, 2024, 12( 8): 1633. DOI: 10.3390/biomedicines12081633. [39] ZENG BT, WU RF, CHEN YS, et al. FTO knockout in adipose tissue effectively alleviates hepatic steatosis partially via increasing the secretion of adipocyte-derived IL-6[J]. Gene, 2022, 818: 146224. DOI: 10.1016/j.gene.2022.146224. [40] HU Y, FENG Y, ZHANG LC, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m6A on lipogenic mRNAs[J]. RNA Biol, 2020, 17( 7): 930- 942. DOI: 10.1080/15476286.2020.1736868. [41] HEROLD KC, REYNOLDS J, DZIURA J, et al. Exenatide extended release in patients with type 1 diabetes with and without residual insulin production[J]. Diabetes Obes Metab, 2020, 22( 11): 2045- 2054. DOI: 10.1111/dom.14121. [42] CHANG Y, DONG MX, WANG Y, et al. GLP-1 gene-modified human umbilical cord mesenchymal stem cell line improves blood glucose level in type 2 diabetic mice[J]. Stem Cells Int, 2019, 2019: 4961865. DOI: 10.1155/2019/4961865. [43] LI S, WANG XM, ZHANG JL, et al. Exenatide ameliorates hepatic steatosis and attenuates fat mass and FTO gene expression through PI3K signaling pathway in nonalcoholic fatty liver disease[J]. Braz J Med Biol Res, 2018, 51( 8): e7299. DOI: 10.1590/1414-431x20187299. [44] JI FH, FU XH, LI GQ, et al. FTO prevents thyroid cancer progression by SLC7A11 m6A methylation in a ferroptosis-dependent manner[J]. Front Endocrinol(Lausanne), 2022, 13: 857765. DOI: 10.3389/fendo.2022.857765. [45] JIANG TY, XIAO Y, ZHOU JF, et al. Arbutin alleviates fatty liver by inhibiting ferroptosis via FTO/SLC7A11 pathway[J]. Redox Biol, 2023, 68: 102963. DOI: 10.1016/j.redox.2023.102963. [46] WANG L, FENG YT, WANG JW, et al. Arbutin ameliorates murine colitis by inhibiting JAK2 signaling pathway[J]. Front Pharmacol, 2021, 12: 683818. DOI: 10.3389/fphar.2021.683818. [47] PENG SM, XIAO W, JU DP, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1[J]. Sci Transl Med, 2019, 11( 488): eaau7116. DOI: 10.1126/scitranslmed.aau7116. [48] VOLLERT J, WANG RS, REGIS S, et al. Genotypes of pain and analgesia in a randomized trial of irritable bowel syndrome[J]. Front Psychiatry, 2022, 13: 842030. DOI: 10.3389/fpsyt.2022.842030. [49] FAN CY, HU HT, HUANG XY, et al. Betaine supplementation causes an increase in fatty acid oxidation and carbohydrate metabolism in livers of mice fed a high-fat diet: A proteomic analysis[J]. Foods, 2022, 11( 6): 881. DOI: 10.3390/foods11060881. [50] SUN LM, GAO M, QIAN QH, et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m6A methylation level to cause lipid metabolism disorder in zebrafish[J]. Sci Total Environ, 2021, 770: 145285. DOI: 10.1016/j.scitotenv.2021.145285. [51] WANG XX, ZHU LN, CHEN JQ, et al. mRNA m6A methylation downregulates adipogenesis in porcine adipocytes[J]. Biochem Biophys Res Commun, 2015, 459( 2): 201- 207. DOI: 10.1016/j.bbrc.2015.02.048. [52] CHENG LD, YU P, LI FF, et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression[J]. Hum Cell, 2021, 34( 6): 1697- 1708. DOI: 10.1007/s13577-021-00593-1. [53] MOZES M, GANTSETSEG G, MANZÉGER A, et al.#5503 Pioglitazone reverses miR-130A and miR-199 dysregulation induced by tgf-beta during kidney fibrosis[J]. Nephrol Dial Transplant, 2023, 38( Suppl 1): i476- i477. DOI: 10.1093/ndt/gfad063c_5503. [54] AN J, CHENG LJ, YANG LP, et al. P-hydroxybenzyl alcohol alleviates oxidative stress in a nonalcoholic fatty liver disease larval zebrafish model and a BRL-3A hepatocyte via the Nrf2 pathway[J]. Front Pharmacol, 2021, 12: 646239. DOI: 10.3389/fphar.2021.646239. -



 PDF下载 ( 908 KB)
PDF下载 ( 908 KB)

 下载:
下载:


