基于肌少症的慢加急性肝衰竭患者90天死亡风险预测模型的建立及验证
DOI: 10.12449/JCH250620
Establishment and validation of a risk prediction model for 90-day mortality in patients with acute-on-chronic liver failure based on sarcopenia
-
摘要:
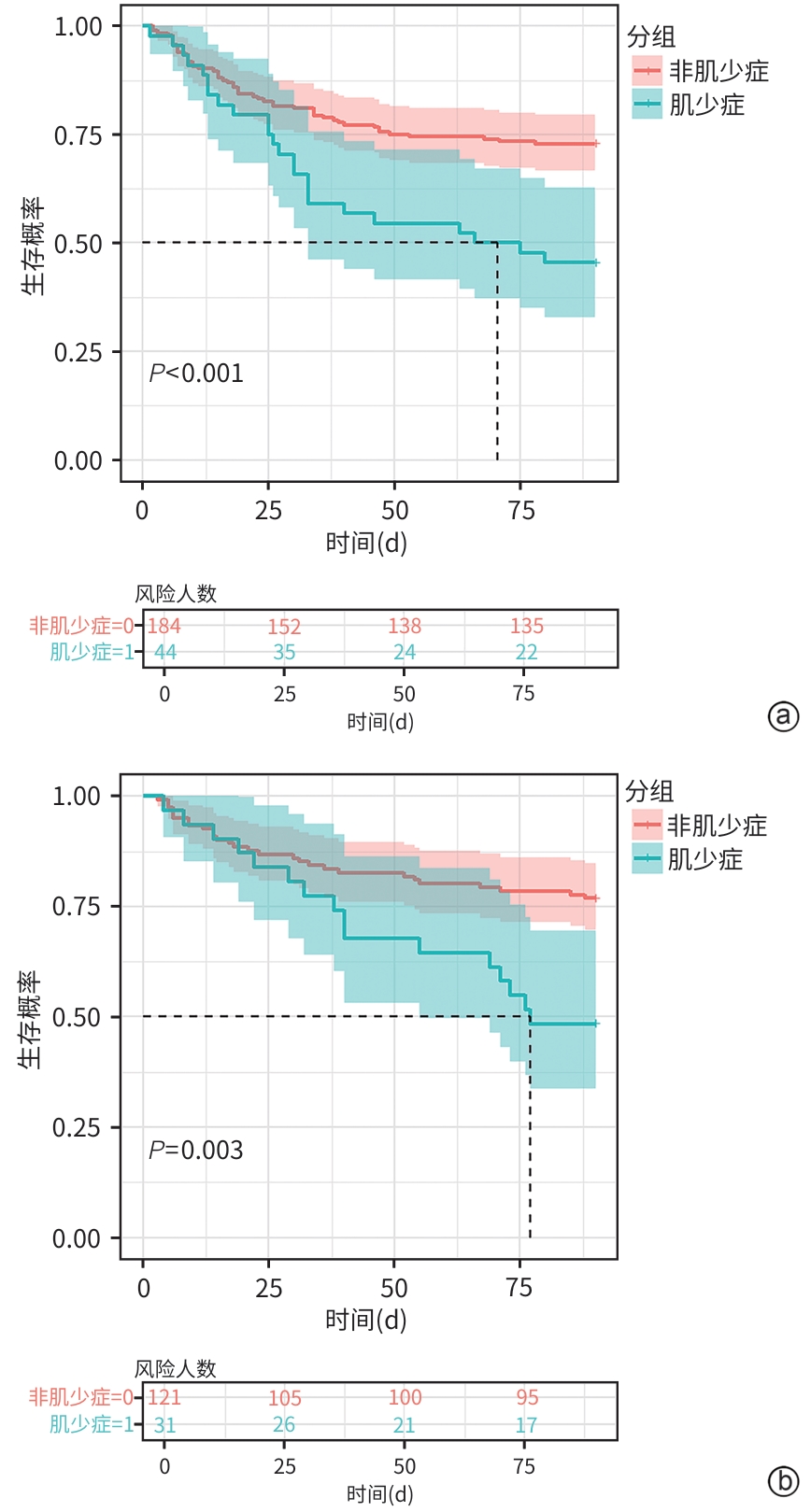
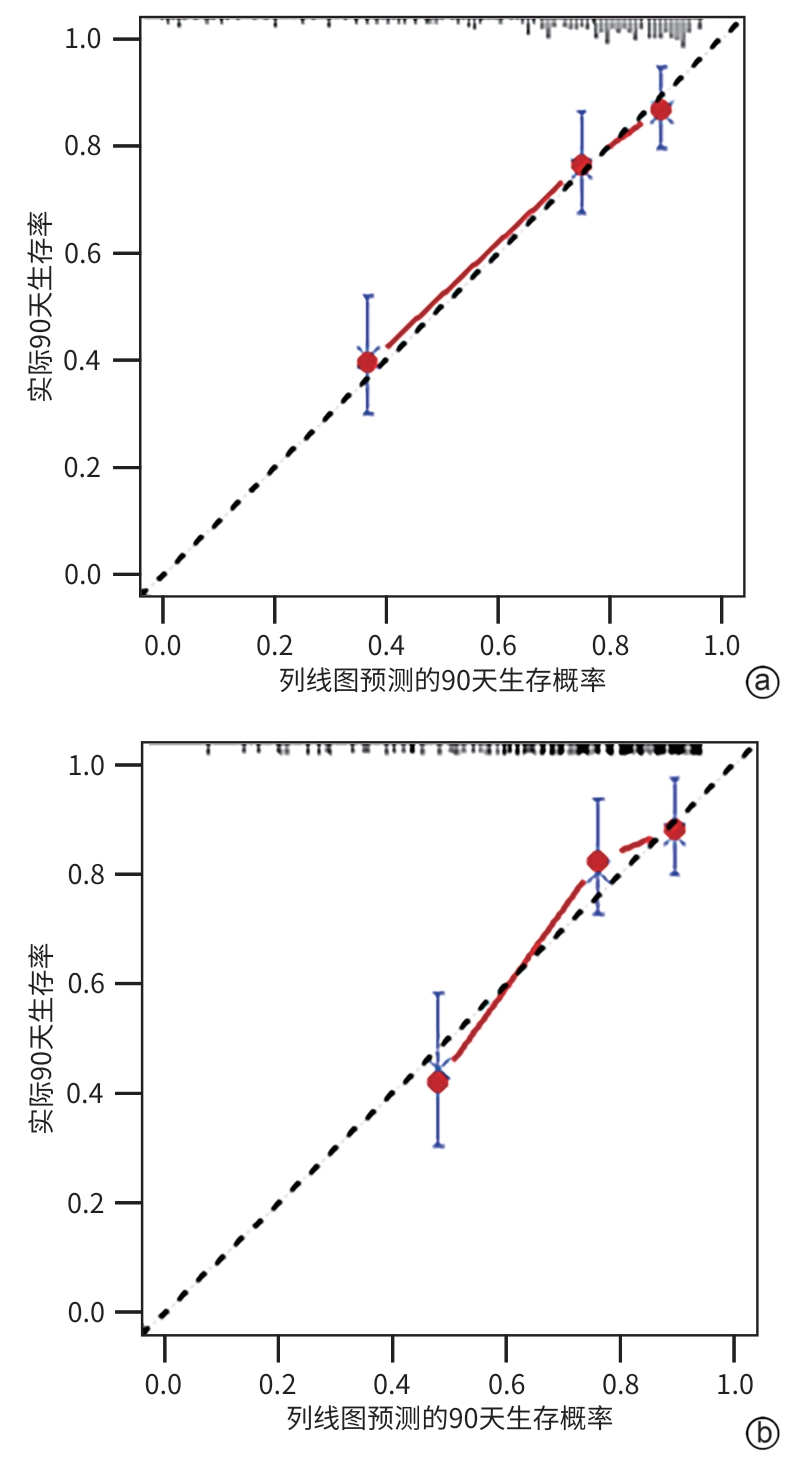
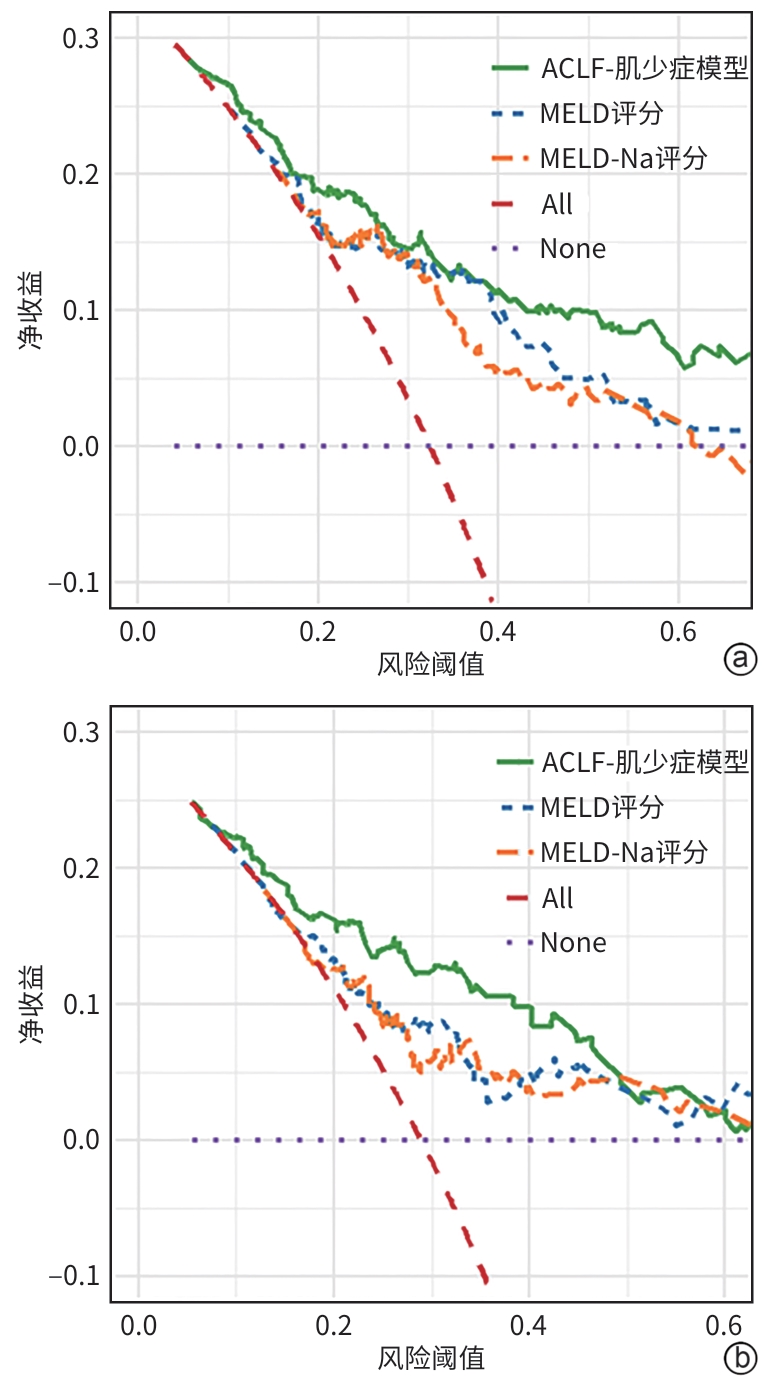
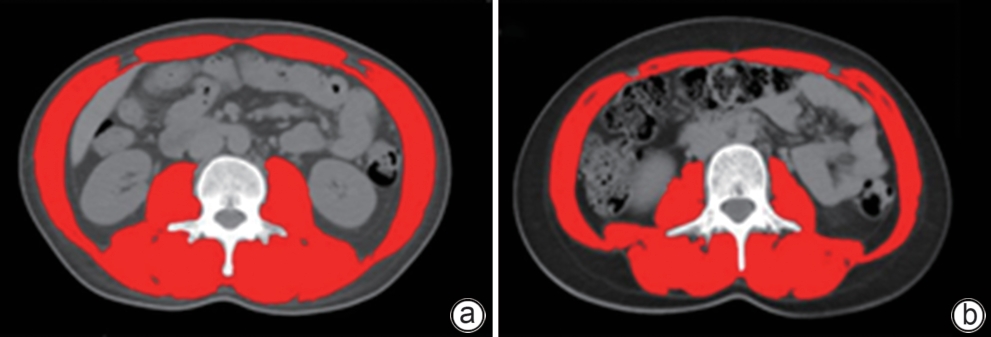
目的 旨在结合肌少症及其他临床指标,构建并验证一个慢加急性肝衰竭(ACLF)患者死亡风险的新预测模型,以提高对ACLF患者预后评估的准确性。 方法 选取2019年1月—2022年1月于首都医科大学附属北京佑安医院住院的ACLF患者380例,采用分层随机抽样法按照6∶4的比例将其分为训练组(n=228)和测试组(n=152)。在训练组中,通过CT图像测量第三腰椎骨骼肌面积,计算第三腰椎骨骼肌指数(L3-SMI)。肌少症的诊断依据前期多中心研究建立的中国北方正常成年人L3-SMI参考值。采用单因素和多因素Cox回归分析,构建结合肌少症及临床风险因素的“肌少症-ACLF模型”,并通过列线图展示。采用受试者操作特征曲线下面积(AUC)评估模型的预测效能,使用校准曲线评估模型的校准度,使用决策曲线分析(DCA)评估其临床应用价值。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用Kaplan-Meier方法绘制生存曲线,组间比较使用Log-rank检验。不同模型间AUC的差异比较采用DeLong检验。 结果 根据多因素Cox回归分析结果,将肌少症(HR=1.962,95%CI:1.185~3.250,P=0.009)、总胆红素(HR=1.003,95%CI:1.002~1.005,P<0.001)、国际标准化比值(HR=1.997,95%CI:1.674~2.382,P<0.001)和乳酸(HR=1.382,95%CI:1.170~1.632,P<0.001)纳入肌少症-ACLF模型。训练队列中,肌少症-ACLF模型预测ACLF患者90天死亡风险的AUC为0.80,较MELD-Na评分的AUC(0.73)有所提高(Z=1.97,P=0.049)。测试队列中,肌少症-ACLF模型的AUC为0.79,显著高于MELD评分(AUC=0.69)(Z=2.70,P=0.007)和MELD-Na评分(AUC=0.68)(Z=2.92,P=0.004)。校准曲线显示该模型具有良好的校准能力,预测的死亡风险与实际观察结果之间一致性较好。DCA结果显示,在一定的阈值概率范围内,训练队列和测试队列中的肌少症-ACLF模型均表现出较MELD评分和MELD-Na评分更高的净收益。 结论 本研究开发的肌少症-ACLF模型为预测ACLF患者90天死亡风险提供了更准确的工具,可支持临床决策和优化治疗策略。 Abstract:Objective To establish and validate a new prediction model for the risk of death in patients with acute-on-chronic liver failure (ACLF) based on sarcopenia and other clinical indicators, and to improve the accuracy of prognostic assessment for ACLF patients. Methods A total of 380 patients with ACLF who were admitted to Beijing YouAn Hospital, Capital Medical University, from January 2019 to January 2022 were enrolled, and they were divided into training group with 228 patients and testing group with 152 patients in a ratio of 6∶4 using the stratified random sampling method. For the training group, CT images were used to measure the cross-sectional area of the skeletal muscle at the third lumbar vertebra (L3), and L3 skeletal muscle index (L3-SMI) was calculated. Sarcopenia was diagnosed based on the previously established L3-SMI reference values for healthy adults in northern China. Univariate and multivariable Cox regression analyses were used to establish a sarcopenia-ACLF model which integrated sarcopenia and clinical risk factors, and a nomogram was developed for presentation. The area under the ROC curve (AUC) was used to assess the predictive performance of the model, the calibration curve was used to assess the degree of calibration, and a decision curve analysis was used to investigate the clinical application value of the model. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. The Kaplan-Meier method was used to plot survival curves, and the Log-rank test was used for comparison between groups. The DeLong test was used for comparison of AUC between different models. Results The multivariate Cox regression analysis showed that sarcopenia (hazard ratio [HR]=1.962, 95% confidence interval [CI]: 1.185 — 3.250, P=0.009), total bilirubin (HR=1.003, 95%CI: 1.002 — 1.005, P<0.001), international normalized ratio (HR=1.997, 95%CI: 1.674 — 2.382, P<0.001), and lactic acid (HR=1.382, 95%CI: 1.170 — 1.632, P<0.001) were included in the sarcopenia-ACLF model. In the training cohort, the sarcopenia-ACLF model had a larger AUC than MELD-Na score in predicting 90-day mortality in patients with ACLF (0.80 vs 0.73, Z=1.97, P=0.049). In the test cohort, the sarcopenia-ACLF model had a significantly larger AUC than MELD score (0.79 vs 0.69, Z=2.70, P=0.007) and MELD-Na score (0.79 vs 0.68, Z=2.92, P=0.004). The calibration curve showed that the model had good calibration ability, with a relatively good consistency between the predicted risk of mortality and the observed results. The DCA results showed that within a reasonable range of threshold probabilities, the sarcopenia-ACLF model showed a greater net benefit than MELD and MELD-Na scores in both the training cohort and the test cohort. Conclusion The sarcopenia-ACLF model developed in this study provides a more accurate tool for predicting the risk of 90-day mortality in ACLF patients, which provides support for clinical decision-making and helps to optimize treatment strategies. -
Key words:
- Acute-On-Chronic Liver Failure /
- Sarcopenia /
- Prognosis
-
表 1 训练队列和测试队列患者的基线特征比较
Table 1. Comparison of baseline characteristics between training and testing cohorts
项目 训练组(n=228) 测试组(n=152) P值 年龄(岁) 47.0(38.0~53.2) 47.5(38.0~54.0) 0.76 年龄分组[例(%)] 0.71 20~<40岁 69(30.3) 43(28.3) 40~60岁 132(57.9) 94(61.8) >60岁 27(11.8) 15(9.9) 性别[例(%)] 0.24 男 188(82.5) 133(87.5) 女 40(17.5) 19(12.5) 病因[例(%)] 0.77 乙型肝炎 124(54.4) 76(50.0) 酒精性肝病 59(25.9) 44(28.9) 乙型肝炎+酒精性肝病 23(10.1) 14(9.2) 其他肝病 22(9.6) 18(11.8) 身高(cm) 172(167~175) 172(167~177) 0.48 体质量(kg) 70.0(63.4~80.0) 73.0(65.0~80.0) 0.11 校正后BMI(kg/m2) 22.9(20.6~25.3) 23.3(20.9~25.4) 0.51 肥胖[例(%)] 66(28.9) 47(30.9) 0.77 肝硬化[例(%)] 180(78.9) 116(76.3) 0.63 腹水[例(%)] 173(75.9) 123(80.9) 0.30 肝性脑病[例(%)] 56(24.6) 31(20.4) 0.41 急性肾损伤[例(%)] 40(17.5) 25(16.4) 0.89 感染[例(%)] 178(78.1) 121(79.6) 0.82 HBV DNA(log10 IU/mL) 4.81(3.51~6.50) 4.61(3.35~6.22) 0.63 乳酸(mmol/L) 2.07(1.71~2.78) 2.05(1.81~2.67) 0.95 器官衰竭等级[例(%)] 0.80 1级 32(14.0) 25(16.4) 2级 125(54.8) 80(52.6) 3级 71(31.1) 47(30.9) 总胆红素(μmol/L) 343(231~450) 318(214~457) 0.54 白蛋白(g/L) 29.01±4.93 28.92±4.95 0.86 肌酐(μmol/L) 61.0(50.0~75.2) 63.5(51.8~76.0) 0.50 钠(mmol/L) 136(132~138) 136(133~139) 0.11 国际标准化比值 2.21(1.93~2.73) 2.16(1.77~2.77) 0.10 血红蛋白(g/L) 121(99~137) 118(103~135) >0.05 白细胞计数(×109/L) 6.81(4.80~9.36) 6.57(5.03~9.57) 0.97 血小板计数(×109/L) 104(70~146) 91(62~144) 0.27 L3-SMI(cm2/m2) 46.20±8.85 46.83±9.14 0.51 肌少症[例(%)] 44(19.3) 31(20.4) 0.90 AARC等级[例(%)] 0.30 1级 38(16.7) 35(23.0) 2级 139(61.0) 87(57.2) 3级 51(22.4) 30(19.7) MELD评分(分) 23.3(20.0~26.9) 22.9(20.1~26.5) 0.60 MELD-Na评分(分) 24.6(21.1~32.1) 24.7(20.6~29.1) 0.48 注:AARC,亚太肝病学会ACLF研究联盟评分。
表 2 单因素Cox回归分析ACLF患者预后的影响因素
Table 2. Univariate Cox regression analysis of prognostic factors in ACLF patients
变量 HR 95%CI P值 年龄 1.020 0.998~1.042 0.072 腹水 2.315 1.189~4.510 0.014 肝性脑病 2.847 1.787~4.534 <0.001 急性肾损伤 2.275 1.372~3.774 0.002 乳酸 1.381 1.206~1.580 <0.001 器官衰竭等级2 3.141 0.963~10.244 0.058 器官衰竭等级3 8.443 2.603~27.379 <0.001 总胆红素 1.003 1.001~1.004 <0.001 钠 0.966 0.935~0.997 0.032 国际标准化比值 1.893 1.602~2.237 <0.001 白细胞 1.052 1.012~1.094 0.011 肌少症 2.287 1.404~3.725 <0.001 AARC等级2 6.751 1.635~27.872 0.008 AARC等级3 18.567 4.425~77.904 <0.001 表 3 各预测模型在训练队列和测试队列中的预测性能比较
Table 3. Comparison of predictive performance of different models in the training and testing cohorts
模型 训练队列 测试队列 AUC(95%CI) P值1) AUC(95%CI) P值1) 肌少症-ACLF
模型0.80
0.74~0.86)0.79
(0.71~0.87)MELD评分 0.74
(0.67~0.82)0.080 0.69
(0.60~0.79)0.007 MELD-Na评分 0.73
(0.66~0.80)0.049 0.68
(0.58~0.78)0.004 注:1)与肌少症-ACLF模型比较。
-
[1] BARKER LA, GOUT BS, CROWE TC. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system[J]. Int J Environ Res Public Health, 2011, 8( 2): 514- 527. DOI: 10.3390/ijerph8020514. [2] CORREIA MI, WAITZBERG DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis[J]. Clin Nutr, 2003, 22( 3): 235- 239. DOI: 10.1016/s0261-5614(02)00215-7. [3] ALLARD JP, KELLER H, JEEJEEBHOY KN, et al. Malnutrition at hospital admission-contributors and effect on length of stay: A prospective cohort study from the Canadian malnutrition task force[J]. JPEN J Parenter Enteral Nutr, 2016, 40( 4): 487- 497. DOI: 10.1177/0148607114567902. [4] CRUZ-JENTOFT AJ, BAHAT G, BAUER J, et al. Sarcopenia: Revised European consensus on definition and diagnosis[J]. Age Ageing, 2019, 48( 1): 16- 31. DOI: 10.1093/ageing/afy169. [5] CEDERHOLM T, BARAZZONI R, AUSTIN P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition[J]. Clin Nutr, 2017, 36( 1): 49- 64. DOI: 10.1016/j.clnu.2016.09.004. [6] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association. Clinical guidelines on nutrition in end-stage liver disease[J]. J Clin Hepatol, 2019, 35( 6): 1222- 1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010.中华医学会肝病学分会, 中华医学会消化病学分会. 终末期肝病临床营养指南[J]. 临床肝胆病杂志, 2019, 35( 6): 1222- 1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010. [7] GENG N, KONG M, CHEN Y, et al. Value of L3 skeletal muscle index in nutritional diagnosis of end-stage liver disease[J]. J Clin Hepatol, 2021, 37( 10): 2493- 2496. DOI: 10.3969/j.issn.1001-5256.2021.10.050.耿楠, 孔明, 陈煜, 等. 第三腰椎骨骼肌指数在终末期肝病营养诊断中的应用价值[J]. 临床肝胆病杂志, 2021, 37( 10): 2493- 2496. DOI: 10.3969/j.issn.1001-5256.2021.10.050. [8] KAMACHI S, MIZUTA T, OTSUKA T, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment[J]. Hepatol Res, 2016, 46( 2): 201- 208. DOI: 10.1111/hepr.12562. [9] KONG M, GENG N, ZHOU Y, et al. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: A multicentre study[J]. Clin Nutr, 2022, 41( 2): 396- 404. DOI: 10.1016/j.clnu.2021.12.003. [10] CURCIO F, TESTA G, LIGUORI I, et al. Sarcopenia and heart failure[J]. Nutrients, 2020, 12( 1): E211. DOI: 10.3390/nu12010211. [11] SHACHAR SS, WILLIAMS GR, MUSS HB, et al. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review[J]. Eur J Cancer, 2016, 57: 58- 67. DOI: 10.1016/j.ejca.2015.12.030. [12] KIM G, KANG SH, KIM MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis[J]. PLoS One, 2017, 12( 10): e0186990. DOI: 10.1371/journal.pone.0186990. [13] SPRINGER J, SPRINGER JI, ANKER SD. Muscle wasting and sarcopenia in heart failure and beyond: Update 2017[J]. ESC Heart Fail, 2017, 4( 4): 492- 498. DOI: 10.1002/ehf2.12237. [14] JOGLEKAR S, ASGHAR A, MOTT SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma[J]. J Surg Oncol, 2015, 111( 6): 771- 775. DOI: 10.1002/jso.23862. [15] O’BRIEN A, WILLIAMS R. Nutrition in end-stage liver disease: Principles and practice[J]. Gastroenterology, 2008, 134( 6): 1729- 1740. DOI: 10.1053/j.gastro.2008.02.001. [16] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [17] CAREY EJ, LAI JC, WANG CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease[J]. Liver Transpl, 2017, 23( 5): 625- 633. DOI: 10.1002/lt.24750. [18] SHI Y, YANG Y, HU YR, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults[J]. Hepatology, 2015, 62( 1): 232- 242. DOI: 10.1002/hep.27795. [19] LI H, CHEN LY, ZHANG NN, et al. Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B[J]. Sci Rep, 2016, 6: 25487. DOI: 10.1038/srep25487. [20] NISHIKAWA H, SHIRAKI M, HIRAMATSU A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease(1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria[J]. Hepatol Res, 2016, 46( 10): 951- 963. DOI: 10.1111/hepr.12774. [21] AKAIKE H. A new look at the statistical model identification[J]. IEEE Trans Autom Contr, 1974, 19( 6): 716- 723. DOI: 10.1109/TAC.1974.1100705. [22] MONTANO-LOZA AJ, MEZA-JUNCO J, PRADO CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis[J]. Clin Gastroenterol Hepatol, 2012, 10( 2): 166- 173. DOI: 10.1016/j.cgh.2011.08.028. [23] HANAI T, SHIRAKI M, WATANABE S, et al. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis[J]. Hepatol Res, 2017, 47( 13): 1359- 1367. DOI: 10.1111/hepr.12873. [24] DASARATHY S. Cause and management of muscle wasting in chronic liver disease[J]. Curr Opin Gastroenterol, 2016, 32( 3): 159- 165. DOI: 10.1097/MOG.0000000000000261. [25] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33( 2): 464- 470. DOI: 10.1053/jhep.2001.22172. [26] BERNAL W, DONALDSON N, WYNCOLL D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: A cohort study[J]. Lancet, 2002, 359( 9306): 558- 563. DOI: 10.1016/S0140-6736(02)07743-7. [27] HERNAEZ R, SOLÀ E, MOREAU R, et al. Acute-on-chronic liver failure: An update[J]. Gut, 2017, 66( 3): 541- 553. DOI: 10.1136/gutjnl-2016-312670. [28] MOURTZAKIS M, PRADO CMM, LIEFFERS JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care[J]. Appl Physiol Nutr Metab, 2008, 33( 5): 997- 1006. DOI: 10.1139/H08-075. [29] SHEN W, PUNYANITYA M, WANG ZM, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image[J]. J Appl Physiol(1985), 2004, 97( 6): 2333- 2338. DOI: 10.1152/japplphysiol.00744.2004. [30] PRADO CMM, HEYMSFIELD SB. Lean tissue imaging: A new era for nutritional assessment and intervention[J]. JPEN J Parenter Enteral Nutr, 2014, 38( 8): 940- 953. DOI: 10.1177/0148607114550189. [31] CLEMENTE-SUÁREZ VJ, REDONDO-FLÓREZ L, BELTRÁN-VELASCO AI, et al. The role of adipokines in health and disease[J]. Biomedicines, 2023, 11( 5): 1290. DOI: 10.3390/biomedicines11051290. [32] EBADI M, MONTANO-LOZA AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis[J]. Dig Liver Dis, 2019, 51( 11): 1493- 1499. DOI: 10.1016/j.dld.2019.05.034. [33] CHEN LK, WOO J, ASSANTACHAI P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment[J]. J Am Med Dir Assoc, 2020, 21( 3): 300- 307. DOI: 10.1016/j.jamda.2019.12.012. [34] PETOUSI N, TALBOT NP, PAVORD I, et al. Measuring lung function in airways diseases: Current and emerging techniques[J]. Thorax, 2019, 74( 8): 797- 805. DOI: 10.1136/thoraxjnl-2018-212441. -



 PDF下载 ( 2616 KB)
PDF下载 ( 2616 KB)

 下载:
下载: