靶向叶酸受体α(FRα)的程序性细胞死亡受体1敲低型嵌合抗原受体T细胞杀伤肝癌细胞的效果分析
DOI: 10.12449/JCH250619
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:温军业、叶学帅负责课题设计,资料分析,撰写论文;张浚琪、任行、张海强参与收集数据,修改论文;温军业、张海强、叶学帅负责拟定写作思路,指导撰写文章并最后定稿。
Efficacy of chimeric antigen receptor T-cell with programmed cell death-1 knockdown targeting folate receptor alpha in killing hepatoma cells
-
摘要:
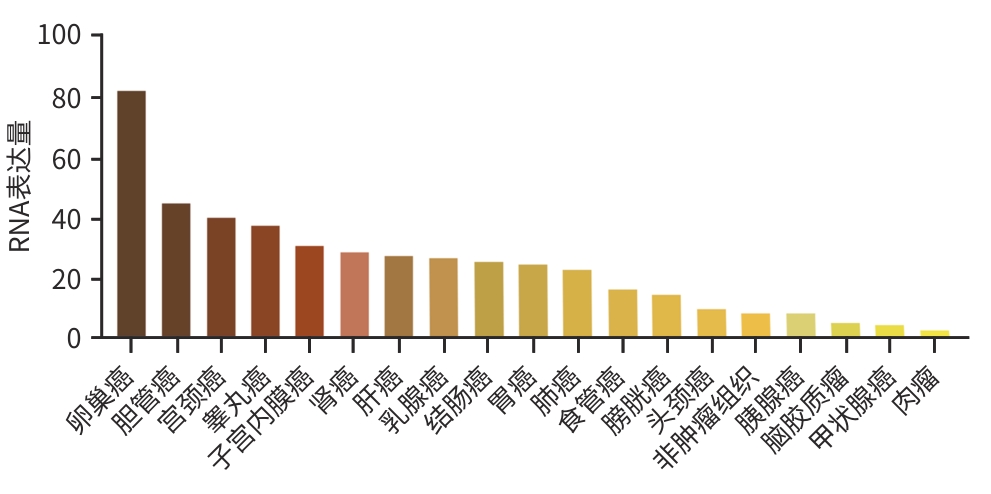
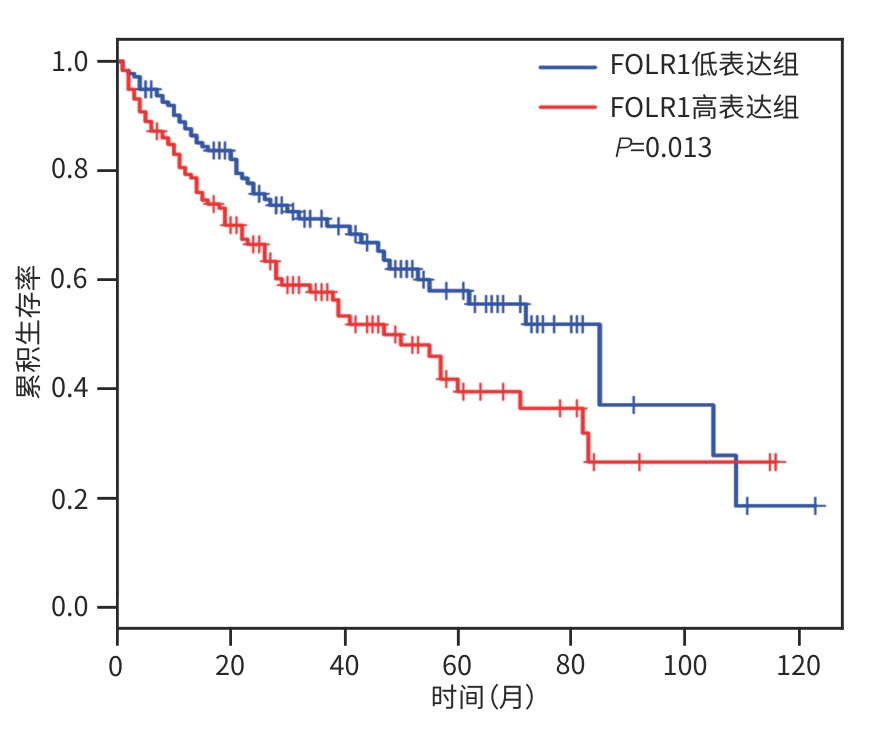
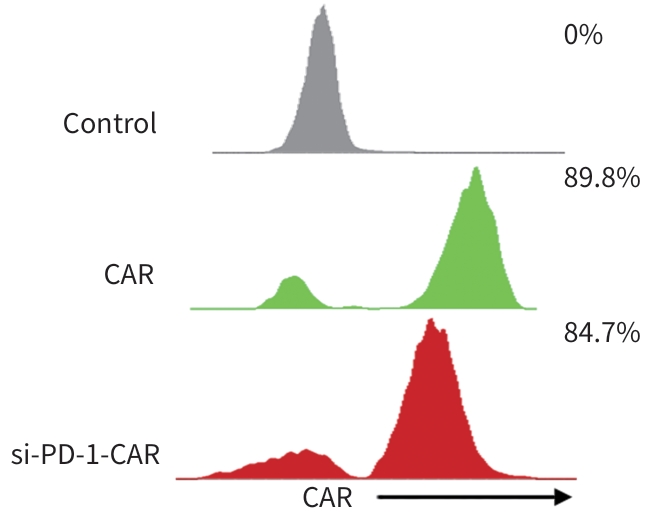
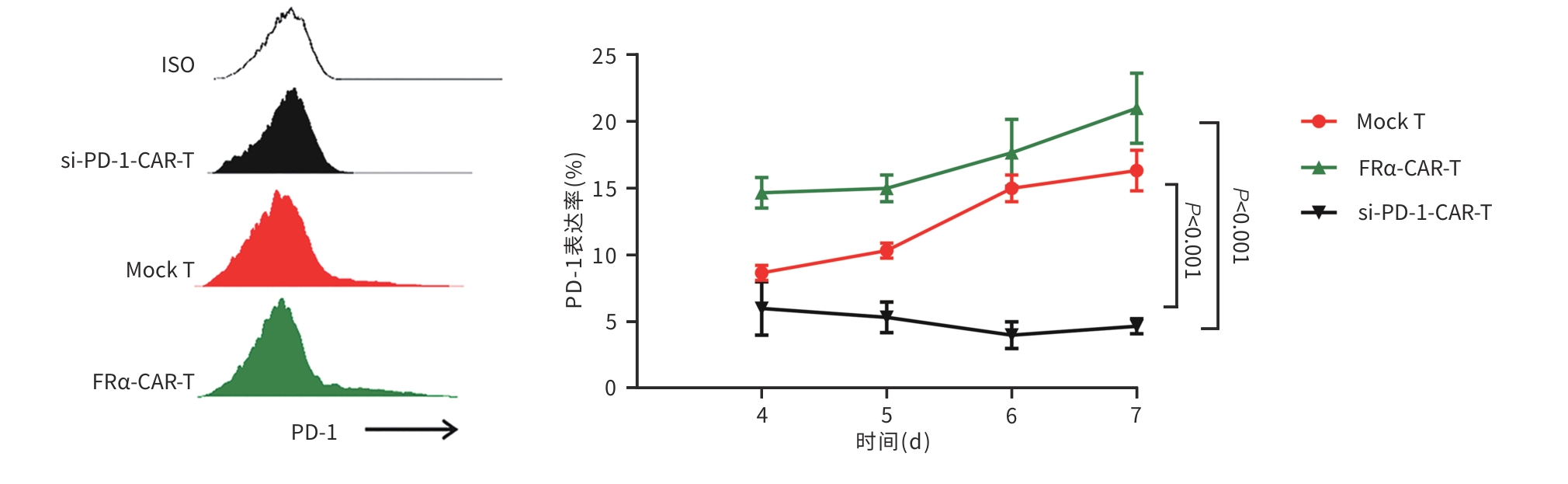
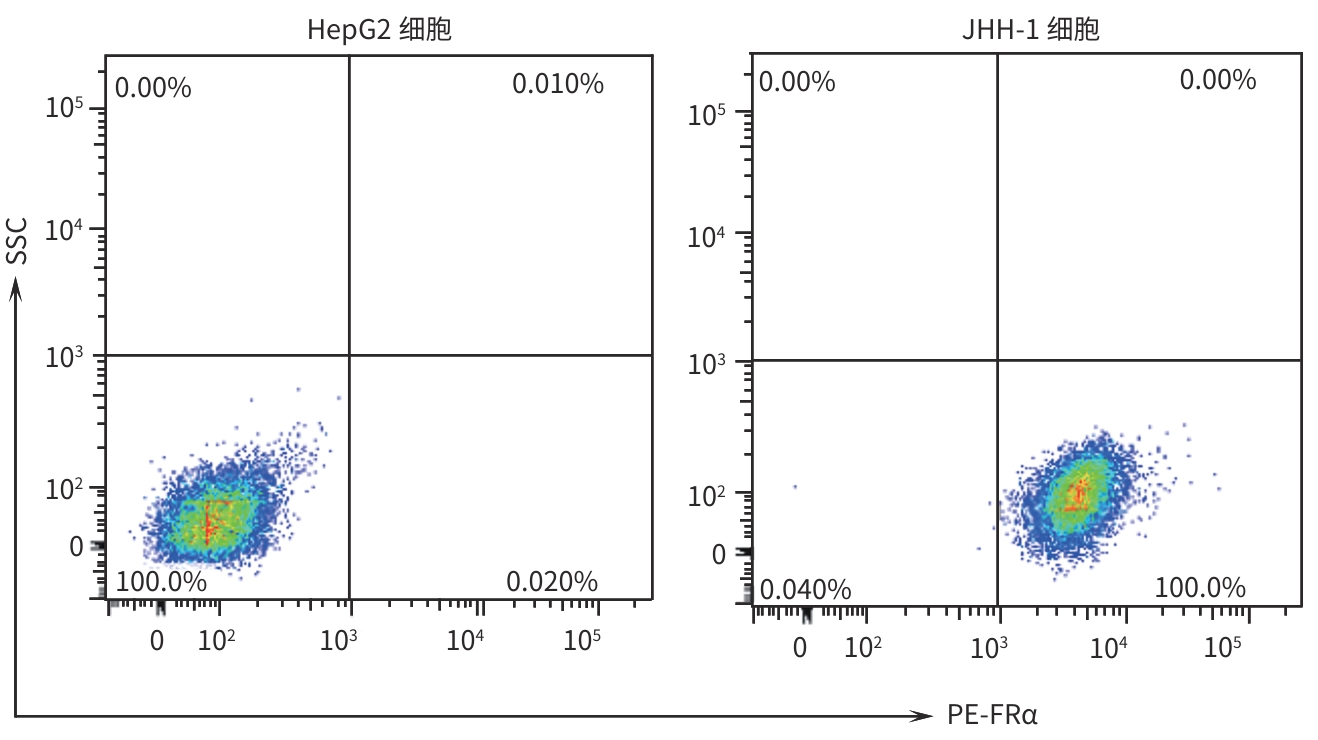
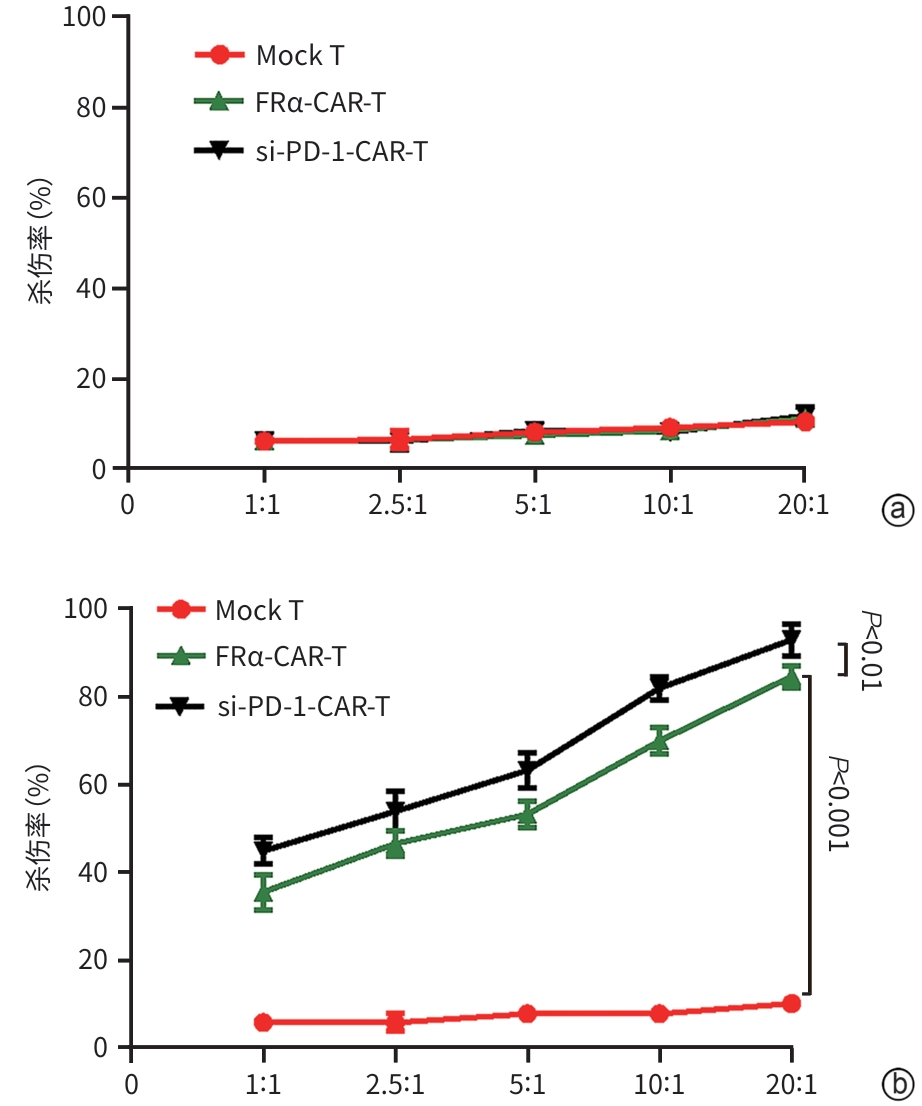
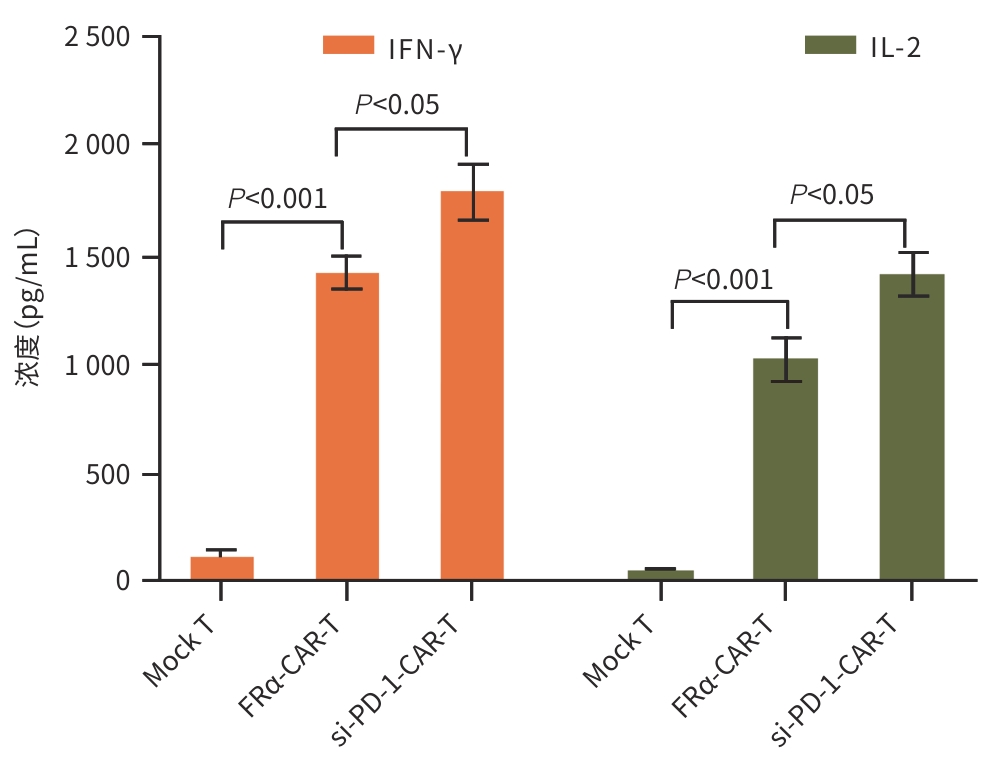
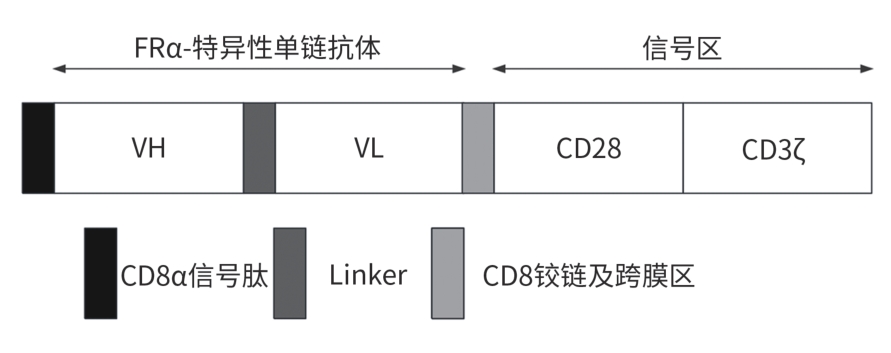
目的 探究靶向叶酸受体α(FRα)嵌合抗原受体(CAR)的程序性细胞死亡受体1(PD-1)敲低型T细胞(si-PD-1-CAR-T)对肝癌细胞的清除能力。 方法 应用生物信息学数据库TCGA分析FRα抗原在肝癌及正常肝组织中的表达情况,以及FRα表达与肝癌患者生存期的关系。分别将编码靶向FRα抗原的CAR结构的mRNA及mRNA联合靶向PD-1基因的小干扰RNA(siRNA)使用电穿孔仪转导入T细胞,制备FRα-CAR-T和si-PD-1-CAR-T。流式细胞术分析FRα-CAR的表达效率和PD-1的敲低效率。体外培养肝癌细胞系JHH-1和HepG2,采用流式细胞术分析FRα在肿瘤细胞表面的表达情况,将FRα-CAR-T、si-PD-1-CAR-T及空载体转导的T细胞(Mock T)作为效应细胞,JHH-1和HepG2作为靶细胞,CCK-8法检测在不同效靶比(1∶1、2.5∶1、5∶1、10∶1、20∶1)时对靶细胞的杀伤效率;采用ELISA法分别检测效应细胞与靶细胞(10∶1)共培养上清中IFN-γ和IL-2的分泌情况。计量资料符合正态分布时,两组间比较采用成组t检验,多组间比较采用单因素方差分析,进一步两两比较采用SNK检验。采用Kaplan-Meier法分析比较患者生存差异。 结果 TCGA数据库分析显示,FOLR1在肝癌组织中表达水平明显升高,FOLR1高表达肝癌患者的总生存期显著低于FOLR1低表达者(P=0.013)。将mRNA转导入T细胞后,FRα-CAR在CAR-T和si-PD-1-CAR-T中的表达率可达89.8%和84.7%,使用mRNA和siRNA共转染可将T细胞的PD-1下调并维持至少7天的PD-1低表达状态。FRα抗原在JHH-1细胞中表达率为100%,而在HepG2细胞中呈阴性表达。CCK-8结果显示,si-PD-1-CAR-T对JHH-1细胞杀伤效率显著高于FRα-CAR-T细胞(P<0.05);ELISA结果显示,FRα-CAR-T细胞与JHH-1细胞共培养时,IL-2分泌量较Mock T细胞显著增加[(1 032.50±135.90) pg/mL vs (50.26±7.87) pg/mL,P<0.001],IFN-γ分泌量显著增加[(1 430.56±184.20) pg/mL vs (89.05±11.26) pg/mL, P<0.001];si-PD-1-CAR-T与JHH-1细胞共培养后,IFN-γ和IL-2的释放水平较FRα-CAR-T均显著提高(P值均<0.05)。 结论 FRα是肝癌治疗的潜在靶点,敲低T细胞PD-1可显著提高FRα-CAR-T在体外的杀伤活性。 -
关键词:
- 癌, 肝细胞 /
- 嵌合抗原受体 /
- 叶酸盐受体1 /
- 程序性细胞死亡受体1
Abstract:Objective To investigate the ability of chimeric antigen receptor T-cell with programmed cell death-1 (PD-1) knockdown (si-PD-1 CAR-T) targeting folate receptor alpha (FRα) to eliminate hepatoma cells. Methods The bioinformatics database TCGA was used to analyze the expression level of FRα antigen in liver cancer tissue and normal liver tissue and the association between FRα expression and the survival of liver cancer patients. The mRNA encoding the CAR structure targeting FRα antigen and the small interfering RNA (siRNA) targeting the PD-1 gene were transduced into T cells using an electroporator to prepare FRα-CAR-T and si-PD-1-CAR-T cells. Flow cytometry was used to analyze the expression efficiency of FRα-CAR and the knockdown efficiency of PD-1. Hepatoma cell lines JHH-1 and Hep-G2 were cultured in vitro, and flow cytometry was used to analyze the expression of FRα on the surface of tumor cells. With FRα-CAR-T, si-PD-1 CAR-T, and mock vector-transduced T cells (Mock T) used as effector cells and with JHH-1 and Hep-G2 cells as target cells, CCK-8 assay was used to measure the killing efficiency of effector cells against target cells at different effector-to-target ratios (1∶1, 2.5∶1,5∶1,10∶1,20∶1). ELISA was used to measure the secretion of interferon gamma (IFN-γ) and interleukin-2 (IL-2) in the supernatants from co-cultures of effector and target cells (10∶1). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, while a one-way analysis of variance was used for comparison between multiple groups, and the SNK test was used for further comparison between two groups. The Kaplan-Meier method was used for comparison of survival differences. Results The analysis of the TCGA database showed that there was a significant increase in the expression level of FOLR1 in liver cancer tissue, and liver cancer patients with high expression of FOLR1 had a significantly shorter overall survival than those with low expression (P=0.013). After transduction of mRNA into T cells, the expression rate of FRα-CAR reached 89.8% in CAR-T and 84.7% in si-PD-1 CAR-T cells, and co-transfection with mRNA and siRNA could downregulate PD-1 in T cells and maintain a low expression state for at least 7 days. The expression rate of FRα antigen was 100% in JHH-1 cells, while it showed negative expression in Hep-G2 cells. CCK-8 assay showed that the killing efficiency of si-PD-1-CAR-T against JHH-1 cells was significantly higher than that against FRα-CAR-T cells (P<0.05). ELISA showed that compared with Mock T cells, FRα-CAR-T cells co-cultured with JHH-1 cells showed significant increases in the secretion of IL-2 (1 032.50±135.90 pg/mL vs 50.26±7.87 pg/mL,P<0.001) and IFN-γ (1 430.56±184.20 pg/mL vs 89.05±11.26 pg/mL,P<0.001), and in addition, the release levels of IFN-γ and IL-2 after co-culture of si-PD-1-CAR-T and JHH-1 cells were significantly higher than the release level of FRα-CAR-T (P<0.05). Conclusion FRα is a potential target for liver cancer treatment, and PD-1 knockdown in T cells can significantly enhance the in vitro killing activity of FRα-CAR-T cells. -
-
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] GANESAN P, KULIK LM. Hepatocellular carcinoma: New developments[J]. Clin Liver Dis, 2023, 27( 1): 85- 102. DOI: 10.1016/j.cld.2022.08.004. [3] COURI T, PILLAI A. Goals and targets for personalized therapy for HCC[J]. Hepatol Int, 2019, 13( 2): 125- 137. DOI: 10.1007/s12072-018-9919-1. [4] SHIMABUKURO-VORNHAGEN A, BÖLL B, SCHELLONGOWSKI P, et al. Critical care management of chimeric antigen receptor T-cell therapy recipients[J]. CA Cancer J Clin, 2022, 72( 1): 78- 93. DOI: 10.3322/caac.21702. [5] DRANSART B, DEHGHANI H, MOORE A. Product-safety considerations in allogeneic chimeric antigen-receptor T-cell process flows[J]. Curr Opin Biotechnol, 2022, 78: 102797. DOI: 10.1016/j.copbio.2022.102797. [6] CARNEIRO BA, EL-DEIRY WS. Targeting apoptosis in cancer therapy[J]. Nat Rev Clin Oncol, 2020, 17( 7): 395- 417. DOI: 10.1038/s41571-020-0341-y. [7] SCARANTI M, COJOCARU E, BANERJEE S, et al. Exploiting the folate receptor α in oncology[J]. Nat Rev Clin Oncol, 2020, 17( 6): 349- 359. DOI: 10.1038/s41571-020-0339-5. [8] GILBERT L, OAKNIN A, MATULONIS UA, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha(FRα)-targeting antibody-drug conjugate(ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer[J]. Gynecol Oncol, 2023, 170: 241- 247. DOI: 10.1016/j.ygyno.2023.01.020. [9] YOUNG O, NGO N, LIN L, et al. Folate receptor as a biomarker and therapeutic target in solid tumors[J]. Curr Probl Cancer, 2023, 47( 1): 100917. DOI: 10.1016/j.currproblcancer.2022.100917. [10] ZHAO YS, DENG J, RAO SF, et al. Tumor infiltrating lymphocyte(TIL) therapy for solid tumor treatment: Progressions and challenges[J]. Cancers(Basel), 2022, 14( 17): 4160. DOI: 10.3390/cancers14174160. [11] HE JJ, XIONG XX, YANG H, et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response[J]. Cell Res, 2022, 32( 6): 530- 542. DOI: 10.1038/s41422-022-00627-9. [12] ZHENG NB, FANG J, XUE G, et al. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance[J]. Cancer Cell, 2022, 40( 9): 973- 985.e7. DOI: 10.1016/j.ccell.2022.08.001. [13] DENLINGER N, BOND D, JAGLOWSKI S. CAR T-cell therapy for B-cell lymphoma[J]. Curr Probl Cancer, 2022, 46( 1): 100826. DOI: 10.1016/j.currproblcancer.2021.100826. [14] YOUNG RM, ENGEL NW, USLU U, et al. Next-generation CAR T-cell therapies[J]. Cancer Discov, 2022, 12( 7): 1625- 1633. DOI: 10.1158/2159-8290.CD-21-1683. [15] XIAO Y, YU DH. Tumor microenvironment as a therapeutic target in cancer[J]. Pharmacol Ther, 2021, 221: 107753. DOI: 10.1016/j.pharmthera.2020.107753. [16] JIANG XJ, WANG J, DENG XY, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape[J]. Mol Cancer, 2019, 18( 1): 10. DOI: 10.1186/s12943-018-0928-4. [17] VESELY MD, ZHANG TX, CHEN LP. Resistance mechanisms to anti-PD cancer immunotherapy[J]. Annu Rev Immunol, 2022, 40: 45- 74. DOI: 10.1146/annurev-immunol-070621-030155. [18] LEKO V, ROSENBERG SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors[J]. Cancer Cell, 2020, 38( 4): 454- 472. DOI: 10.1016/j.ccell.2020.07.013. [19] UPADHYAY R, BOIARSKY JA, PANTSULAIA G, et al. A critical role for fas-mediated off-target tumor killing in T-cell immunotherapy[J]. Cancer Discov, 2021, 11( 3): 599- 613. DOI: 10.1158/2159-8290.CD-20-0756. [20] WANG HZ, YE XS, JU Y, et al. Minicircle DNA-mediated CAR T cells targeting CD44 suppressed hepatocellular carcinoma both in vitro and in vivo[J]. Onco Targets Ther, 2020, 13: 3703- 3716. DOI: 10.2147/OTT.S247836. [21] SUN L, GAO F, GAO ZH, et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in hepatocellular carcinoma[J]. J Immunother Cancer, 2021, 9( 4): e001875. DOI: 10.1136/jitc-2020-001875. [22] PANG NZ, SHI JX, QIN L, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin[J]. J Hematol Oncol, 2021, 14( 1): 118. DOI: 10.1186/s13045-021-01128-9. [23] SAITO S, KOYA Y, KAJIYAMA H, et al. Folate-appended cyclodextrin carrier targets ovarian cancer cells expressing the proton-coupled folate transporter[J]. Cancer Sci, 2020, 111( 5): 1794- 1804. DOI: 10.1111/cas.14379. [24] CHEUNG A, OPZOOMER J, ILIEVA KM, et al. Anti-folate receptor alpha-directed antibody therapies restrict the growth of triple-negative breast cancer[J]. Clin Cancer Res, 2018, 24( 20): 5098- 5111. DOI: 10.1158/1078-0432.CCR-18-0652. [25] LUANGWATTANANUN P, JUNKING M, SUJJITJOON J, et al. Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy[J]. Breast Cancer Res Treat, 2021, 186( 1): 25- 36. DOI: 10.1007/s10549-020-06032-3. [26] NAWAZ FZ, KIPREOS ET. Emerging roles for folate receptor FOLR1 in signaling and cancer[J]. Trends Endocrinol Metab, 2022, 33( 3): 159- 174. DOI: 10.1016/j.tem.2021.12.003. [27] SHARMA A, SEOW JJW, DUTERTRE CA, et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma[J]. Cell, 2020, 183( 2): 377- 394.e21. DOI: 10.1016/j.cell.2020.08.040. [28] ROSELLI E, BOUCHER JC, LI GB, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells[J]. J Immunother Cancer, 2021, 9( 10): e003354. DOI: 10.1136/jitc-2021-003354. [29] MAGNANI CF, GAIPA G, LUSSANA F, et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities[J]. J Clin Invest, 2020, 130( 11): 6021- 6033. DOI: 10.1172/JCI138473. [30] HUANG RH, LI XP, HE YD, et al. Recent advances in CAR-T cell engineering[J]. J Hematol Oncol, 2020, 13( 1): 86. DOI: 10.1186/s13045-020-00910-5. [31] BILLINGSLEY MM, SINGH N, RAVIKUMAR P, et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering[J]. Nano Lett, 2020, 20( 3): 1578- 1589. DOI: 10.1021/acs.nanolett.9b04246. [32] SOUNDARA RAJAN T, GUGLIANDOLO A, BRAMANTI P, et al. In vitro-transcribed mRNA chimeric antigen receptor T cell(IVT mRNA CAR T) therapy in hematologic and solid tumor management: A preclinical update[J]. Int J Mol Sci, 2020, 21( 18): 6514. DOI: 10.3390/ijms21186514. [33] MORETTI A, PONZO M, NICOLETTE CA, et al. The past, present, and future of non-viral CAR T cells[J]. Front Immunol, 2022, 13: 867013. DOI: 10.3389/fimmu.2022.867013. -



 PDF下载 ( 2721 KB)
PDF下载 ( 2721 KB)

 下载:
下载: