经颈静脉肝内门体分流术治疗肝硬化伴食管胃静脉曲张破裂出血患者术后生存预测模型的建立和验证
DOI: 10.12449/JCH250618
Establishment and validation of a predictive model for survival after transjugular intrahepatic portosystemic shunt in patients with liver cirrhosis and esophagogastric variceal bleeding
-
摘要:
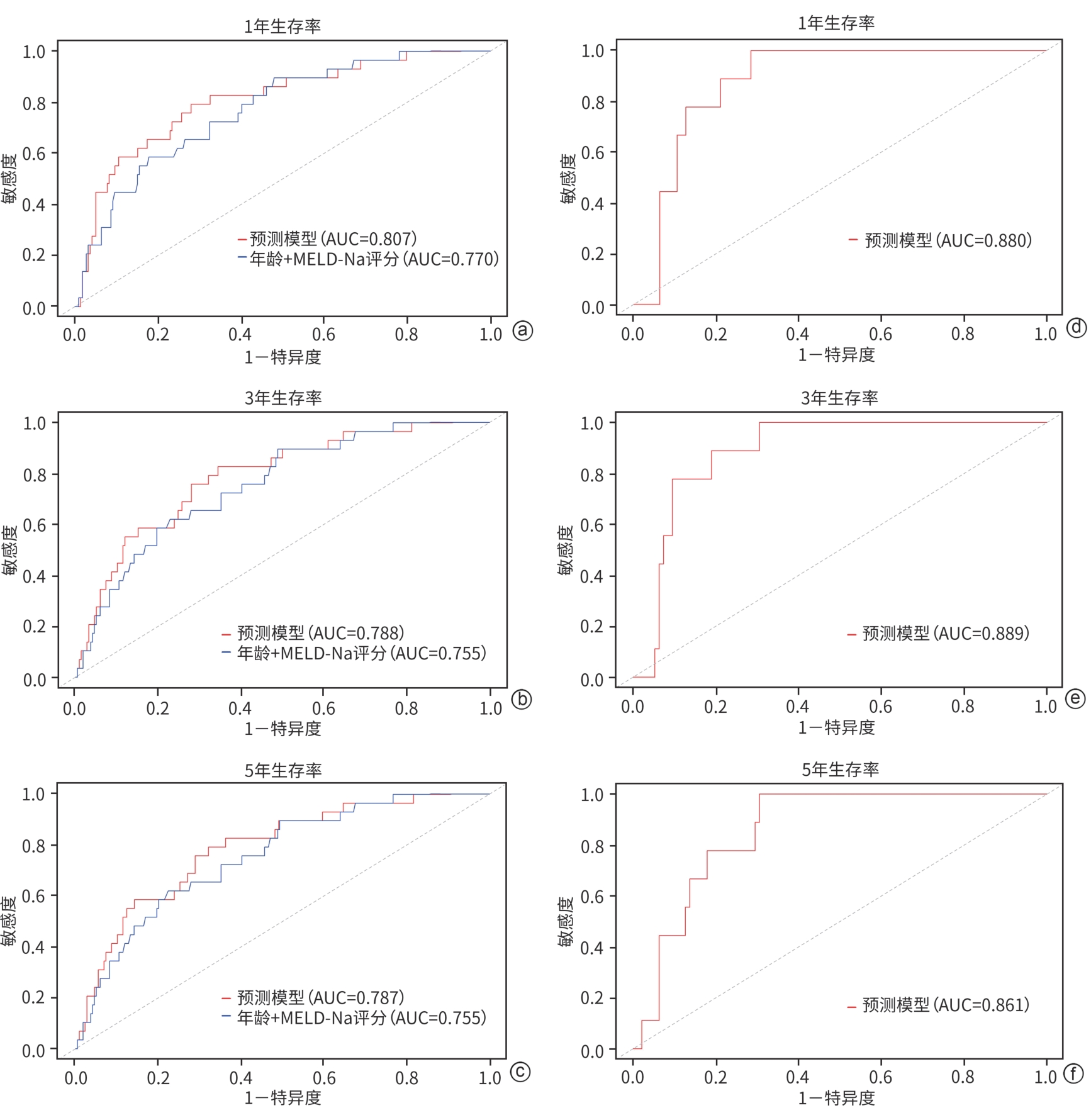
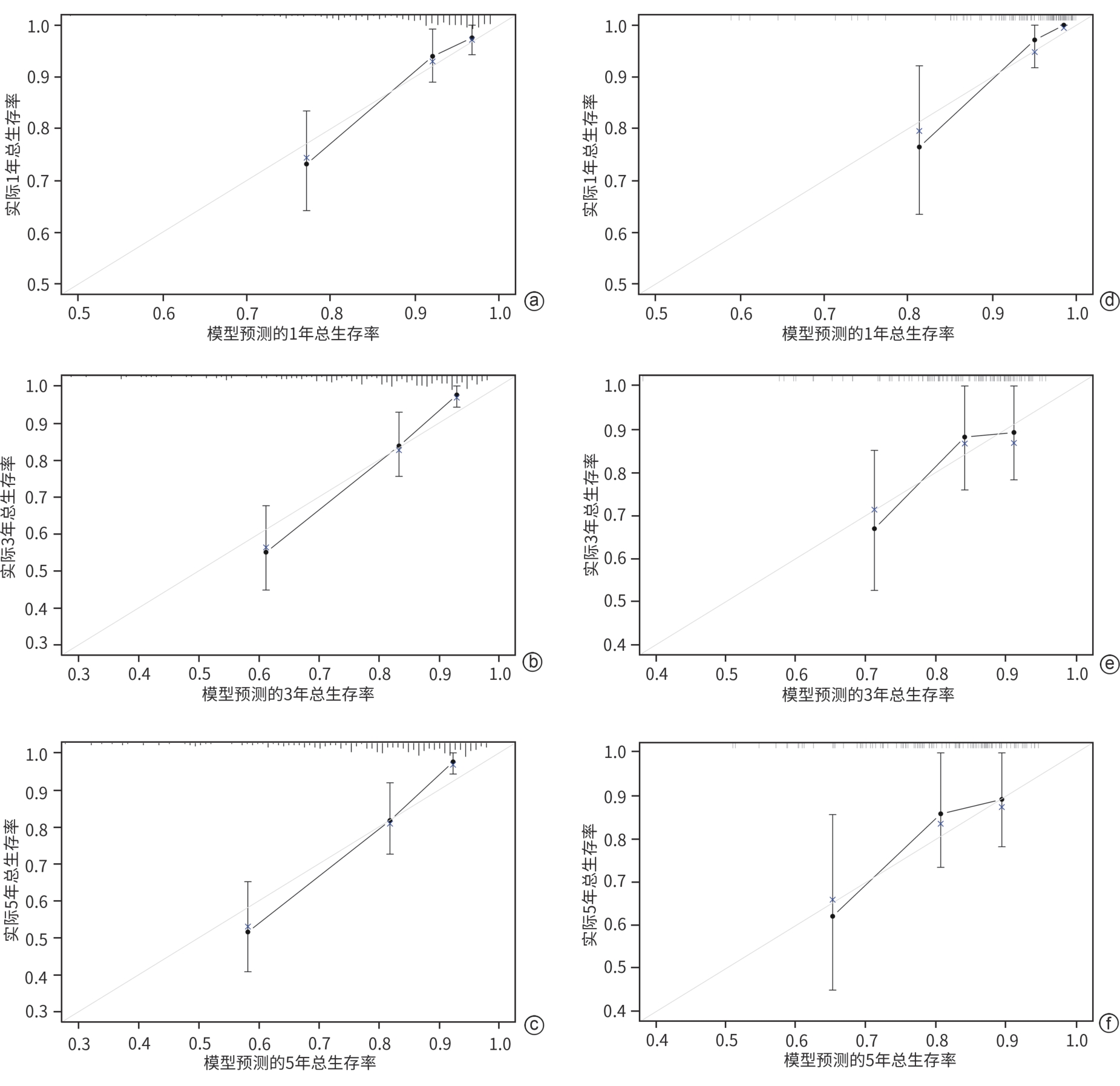
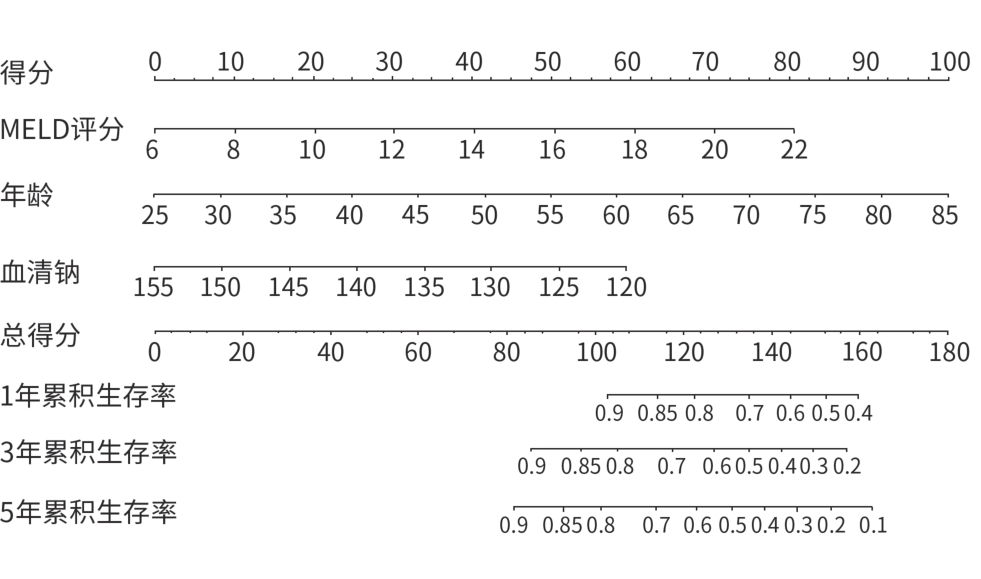
目的 以肝硬化伴食管胃静脉曲张破裂出血(EGVB)患者为研究对象,综合评估经颈静脉肝内门体分流术(TIPS)后生存相关危险因素,构建TIPS术后生存预测模型。 方法 收集2015年1月—2018年12月在南京大学医学院附属鼓楼医院消化内科接受TIPS治疗的352例肝硬化伴EGVB患者的临床资料,并按照7∶3的比例随机分配至训练组(n=248)和验证组(n=104)。采用Cox回归分析筛选出影响TIPS术后生存的独立危险因素,构建预测模型列线图;采用一致性指数(C-index)和受试者操作特征曲线(ROC曲线)评估模型的区分能力,同时通过校准曲线评估模型预测价值。符合正态分布的计量资料两组间比较采用成组t检验;非正态分布的计量资料两组间比较采用Wilcoxon秩和检验。计数资料两组间比较采用χ2检验。通过Kaplan-Meier分析计算累积生存率。 结果 训练组患者的1、3、5年累积生存率分别为91.1%、79.5%和77.0%。Cox多因素回归分析结果显示,年龄(HR=1.047,95%CI:1.032~1.092,P<0.001)、MELD评分(HR=1.127,95%CI:1.003~1.268,P=0.045)和血清钠水平(HR=0.928,95%CI:0.878~0.981,P=0.008)是患者生存的独立影响因素,并以此建立预测模型和列线图。训练组和验证组预测模型C-index分别为0.760和0.757。训练组列线图预测1、3、5年生存率的ROC曲线下面积分别为0.807、0.788和0.787。校准曲线显示列线图预测与实际结果一致性较好。 结论 基于年龄、MELD评分和血清钠构建了预测肝硬化伴EGVB患者TIPS术后生存的列线图模型,该模型具有良好的区分度与准确度。 -
关键词:
- 肝硬化 /
- 食管和胃静脉曲张 /
- 门体分流术, 经颈静脉肝内 /
- 预后 /
- 列线图
Abstract:Objective To investigate the risk factors for survival after transjugular intrahepatic portosystemic shunt (TIPS) in patients with liver cirrhosis and esophagogastric variceal bleeding (EGVB), and to establish a predictive model for survival after TIPS. Methods Clinical data were collected from 352 patients with liver cirrhosis and EGVB who underwent TIPS in Department of Gastroenterology, Affiliated Drum Tower Hospital of Nanjing University Medical School, from January 2015 to December 2018, and the patients were randomly divided into training group (n=248) and validation group (n=104) at a ratio of 7∶3. The Cox regression analysis was used to identify the independent risk factors for survival after TIPS, and a nomogram predictive model was established. The index of concordance (C-index) and the receiver operating characteristic (ROC) curve were used to assess the discriminatory ability of the model, and the calibration curve was used to assess the predictive value of the model. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. The Kaplan-Meier analysis was used to calculate cumulative survival rate. Results For the patients in the training group, the 1-,3-, and 5-year cumulative survival rates were 91.1%,79.5%, and 77.0%, respectively. The multivariate Cox regression analysis showed that age (hazard ratio [HR]=1.047, 95% confidence interval [CI]:1.032 — 1.092,P<0.001), MELD score (HR=1.127,95%CI:1.003 — 1.268,P=0.045), and serum sodium (Na) (HR=0.928,95%CI:0.878 — 0.981,P=0.008) were independent influencing factors for survival, and a predictive model and a nomogram were established based on these factors. The predictive model had a C-index of 0.760 in the training group and 0.757 in the validation group. In the training group, the nomogram had an area under the ROC curve of 0.807,0.788, and 0.787, respectively, in predicting 1-,3-, and 5-year cumulative survival rates. The calibration curve showed relatively high consistency between the results predicted by the nomogram and the actual results. Conclusion A nomogram model is established based on age, MELD score, and Na for predicting survival after TIPS in patients with liver cirrhosis and EGVB, and this model has good discriminatory ability and accuracy. -
表 1 基线特征比较
Table 1. Clinical characteristics of patients
指标 训练组(n=248) 验证组(n=104) 统计值 P值 年龄(岁) 55.25±11.45 58.96±11.59 t=0.765 0.352 男[例(%)] 151(60.9) 65(62.5) χ2=0.080 0.777 Child-Pugh评分(分) 7.34±1.36 7.23±1.23 t=0.723 0.470 Child-Pugh分级(A/B/C,例) 71/164/13 26/76/2 χ2=2.763 0.251 MELD评分(分) 10.33±2.41 10.58±2.30 t=0.900 0.369 MELD-Na评分(分) 10.89±3.97 11.37±4.73 t=0.967 0.334 凝血酶原时间(s) 14.84±1.88 14.92±2.05 t=0.382 0.703 INR 1.29±0.16 1.30±0.18 t=0.491 0.624 纤维蛋白原(g/L) 1.57±0.59 1.60±0.70 t=0.382 0.700 D-二聚体(mg/L) 1.97(0.75~3.96) 1.91(0.64~3.99) Z=0.099 0.921 ALT(U/L) 21.50(15.65~33.10) 18.70(14.15~27.05) Z=1.069 0.285 AST(U/L) 28.70(22.20~39.90) 27.20(20.20~27.20) Z=0.462 0.644 总胆红素(μmol/L) 18.20(12.95~25.75) 18.10(13.10~24.75) Z=0.327 0.744 白蛋白(g/L) 32.55±4.23 32.46±4.51 t=0.176 0.860 SCr(μmol/L) 63.00±19.92 68.06±25.68 t=0.988 0.348 血清钠(μmol/L) 139.91±4.17 139.35±4.95 t=1.134 0.256 白细胞计数(×109/L) 2.90(1.95~4.50) 3.10(1.85~4.95) Z=0.171 0.865 血小板计数(×109/L) 65.00(43.00~114.00) 73.00(45.00~133.50) Z=0.278 0.781 肝硬化病因(例) 病毒性/酒精性/免疫性/其他 158/17/31/42 52/8/12/32 χ2=8.706 0.227 腹水(无/轻/中/重,例) 51/84/84/29 27/36/27/14 χ2=2.616 0.455 脾切除史[例(%)] 54(21.77) 18(17.31) χ2=0.898 0.343 合并糖尿病[例(%)] 41(16.53) 28(26.92) χ2=5.020 0.125 既往HE[例(%)] 9(3.62) 3(2.88) χ2=0.123 0.725 支架直径(6/7/8 mm,例) 57/39/152 29/19/56 χ2=1.695 0.428 注:INR,国际标准化比值;SCr,血清肌酐。
表 2 训练队列单因素和多因素 Cox回归分析结果
Table 2. Univariate and multivariate Cox regression analysis
变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(岁) 1.052(1.025~1.079) <0.001 1.047(1.032~1.092) <0.001 性别 0.621(0.340~1.135) 0.122 肝硬化病因 病毒性 1.000 酒精性 0.685(0.210~2.231) 0.530 免疫性 1.380(0.662~2.878) 0.390 其他 1.238(0.627~2.444) 0.538 Child-Pugh评分(分) 1.440(1.184~1.752) <0.001 1.253(0.964~1.629) 0.091 MELD评分(分) 1.187(1.092~1.291) <0.001 1.127(1.003~1.268) 0.045 MELD-Na评分(分) 1.072(1.033~1.113) <0.001 凝血酶原时间(s) 1.162 (1.011~1.335) 0.035 INR 6.921(1.374~34.865) 0.019 纤维蛋白原(g/L) 0.942(0.559~1.586) 0.821 D-二聚体(mg/L) 1.002(0.968~1.037) 0.907 ALT(U/L) 0.998(0.992~1.005) 0.639 AST(U/L) 0.998(0.981~1.006) 0.656 总胆红素(μmol/L) 1.006(1.000~1.013) 0.040 白蛋白(g/L) 0.919(0.858~0.983) 0.014 SCr(μmol/L) 1.009(0.999~1.020) 0.088 血清钠(μmol/L) 0.922(0.876~0.970) 0.002 0.928(0.878~0.981) 0.008 白细胞计数(×109/L) 1.108(1.035~1.185) 0.003 1.064(0.991~1.142) 0.087 血小板计数(×109/L) 0.999(0.994~1.003) 0.506 既往HE 1.002(0.989~1.016) 0.713 脾切除史 1.316(0.641~2.704) 0.455 合并糖尿病 2.259(1.236~4.129) 0.008 1.806(0.967~3.372) 0.064 Child-Pugh分级 A级 1.000 B级 1.712(0.849~3.450) 0.133 C级 3.783(1.291~11.086) 0.015 腹水 无 1.000 轻 0.756(0.308~1.853) 0.540 中 1.337(0.673~2.655) 0.407 重 2.642(1.207~5.783) 0.015 注:性别赋值:0=女,1=男;肝硬化病因赋值:1=病毒性,2=酒精性,3=免疫学,4=其他;既往HE赋值:0=无,1=有;脾切除史赋值:0=无,1=有;合并糖尿病赋值:0=无,1=有;Child-Pugh分级赋值:0=A级,1=B级,2=C级;腹水赋值:0=无,1=轻,2=中,3=重。
表 3 模型评估
Table 3. Models evaluated
模型 AUC AIC BIC 列线图模型 0.807 293 304 Child-Pugh评分 0.720 302 306 MELD评分 0.702 309 313 FIPS评分 0.713 522 536 CLIF-C AD评分 0.777 296 310 注:AIC,赤池信息准则;BIC,贝叶斯信息准则。CLIF-C AD=10×[(0.03×年龄)+0.66×ln(SCr÷88.4)+(1.71×lnINR)+(0.88×lnWBC)-(0.05×Na)+8],FIPS=1.43×lgTBil-88.4×1.71÷SCr+0.02×年龄-0.02×Alb。
-
[1] de FRANCHIS R, FACULTY BV. Expanding consensus in portal hypertension: Report of the baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension[J]. J Hepatol, 2015, 63( 3): 743- 752. DOI: 10.1016/j.jhep.2015.05.022. [2] GARCIA-TSAO G, ABRALDES JG, BERZIGOTTI A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases[J]. Hepatology, 2017, 65( 1): 310- 335. DOI: 10.1002/hep.28906. [3] NIEKAMP A, KUBAN JD, LEE SR, et al. Transjugular intrahepatic portosystemic shunts reduce variceal bleeding and improve survival in patients with cirrhosis: A population-based analysis[J]. J Vasc Interv Radiol, 2020, 31( 9): 1382- 1391. e 2. DOI: 10.1016/j.jvir.2020.06.005. [4] NICOARĂ-FARCĂU O, HAN GH, RUDLER M, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: A meta-analysis of individual patient data[J]. Gastroenterology, 2021, 160( 1): 193- 205. e 10. DOI: 10.1053/j.gastro.2020.09.026. [5] Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on the diagnosis and treatment of esophageal and gastric variceal rupture bleeding in cirrhotic portal hypertension(2025 edition)[J]. Chin J Dig Surg, 2025, 24( 3): 271- 280. DOI: 10.3760/cma.j.cn115610-20241228-00590.中华医学会外科学分会脾及门静脉高压外科学组. 肝硬化门静脉高压症食管、 胃底静脉曲张破裂出血诊治专家共识(2025版)[J]. 中华消化外科杂志, 2025, 24( 3): 271- 280. DOI: 10.3760/cma.j.cn115610-20241228-00590. [6] NARDELLI S, GIOIA S, RIDOLA L, et al. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis[J]. Hepatology, 2019, 70( 2): 640- 649. DOI: 10.1002/hep.30304. [7] NARDELLI S, LATTANZI B, MERLI M, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis[J]. Hepatology, 2019, 70( 5): 1704- 1713. DOI: 10.1002/hep.30692. [8] SOLÉ C, GUILLY S, SILVA K DA, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: Relationship with acute-on-chronic liver failure and prognosis[J]. Gastroenterology, 2021, 160( 1): 206- 218. e 13. DOI: 10.1053/j.gastro.2020.08.054. [9] GABA RC, COUTURE PM, BUI JT, et al. Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation[J]. J Vasc Interv Radiol, 2013, 24( 3): 411- 420, 420. e1-4; quiz 421. DOI: 10.1016/j.jvir.2012.10.026. [10] TEJEDOR-TEJADA J, FUENTES-VALENZUELA E, GARCÍA-PAJARES F, et al. Long-term clinical outcome and survival predictors in patients with cirrhosis after 10-mm-covered transjugular intrahepatic portosystemic shunt[J]. Gastroenterol Hepatol, 2021, 44( 9): 620- 627. DOI: 10.1016/j.gastrohep.2020.10.018. [11] LATTANZI B, NARDELLI S, PIGLIACELLI A, et al. The additive value of sarcopenia, myosteatosis and hepatic encephalopathy in the predictivity of model for end-stage liver disease[J]. Dig Liver Dis, 2019, 51( 11): 1508- 1512. DOI: 10.1016/j.dld.2019.09.004. [12] CHEN DX, LIU Z, LIU WJ, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram[J]. Nat Commun, 2021, 12( 1): 179. DOI: 10.1038/s41467-020-20429-0. [13] DONG D, TANG L, LI ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer[J]. Ann Oncol, 2019, 30( 3): 431- 438. DOI: 10.1093/annonc/mdz001. [14] ZHANG F, ZHUGE YZ, ZOU XP, et al. Different scoring systems in predicting survival in Chinese patients with liver cirrhosis undergoing transjugular intrahepatic portosystemic shunt[J]. Eur J Gastroenterol Hepatol, 2014, 26( 8): 853- 860. DOI: 10.1097/MEG.0000000000000134. [15] MALINCHOC M, KAMATH PS, GORDON FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts[J]. Hepatology, 2000, 31( 4): 864- 871. DOI: 10.1053/he.2000.5852. [16] ANGERMAYR B, CEJNA M, KARNEL F, et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt[J]. Gut, 2003, 52( 6): 879- 885. DOI: 10.1136/gut.52.6.879. [17] SIBAE MR AL, CAPPELL MS. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS[J]. Dig Dis Sci, 2011, 56( 4): 977- 987. DOI: 10.1007/s10620-010-1390-3. [18] FERRAL H, GAMBOA P, POSTOAK DW, et al. Survival after elective transjugular intrahepatic portosystemic shunt creation: Prediction with model for end-stage liver disease score[J]. Radiology, 2004, 231( 1): 231- 236. DOI: 10.1148/radiol.2311030967. [19] YOUNG S, ROSTAMBEIGI N, GOLZARIAN J, et al. MELD or sodium MELD: A comparison of the ability of two scoring systems to predict outcomes after transjugular intrahepatic portosystemic shunt placement[J]. AJR Am J Roentgenol, 2020, 215( 1): 215- 222. DOI: 10.2214/AJR.19.21726. [20] SAAD N, RUDE MK, DARCY M, et al. Older age is associated with increased early mortality after transjugular intrahepatic portosystemic shunt[J]. Ann Hepatol, 2016, 15( 2): 215- 221. DOI: 10.5604/16652681.1193716. [21] YIN Q, WU ZR, ZHANG F, et al. Risk factors for unplanned readmission after transjugular intrahepatic portosystemic shunt in cirrhotic patients with esophagogastric variceal bleeding and construction of a nomogram model[J]. J Clin Hepatol, 2024, 40( 9): 1796- 1801. DOI: 10.12449/JCH240913.殷芹, 吴兆荣, 张峰, 等. 肝硬化食管胃底静脉曲张破裂出血患者经颈静脉肝内门体分流术后非计划再入院的危险因素分析及列线图模型构建[J]. 临床肝胆病杂志, 2024, 40( 9): 1796- 1801. DOI: 10.12449/JCH240913. [22] YIN XC, ZHANG F, GUO HW, et al. A nomogram to predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients[J]. Sci Rep, 2020, 10( 1): 9381. DOI: 10.1038/s41598-020-65227-2. -



 PDF下载 ( 1714 KB)
PDF下载 ( 1714 KB)

 下载:
下载: