抑制脯氨酰内肽酶表达对非酒精性脂肪性肝炎小鼠模型的影响及作用机制
DOI: 10.12449/JCH250615
Effect of prolyl endopeptidase expression inhibition on a mouse model of non-alcoholic steatohepatitis and its mechanism
-
摘要:
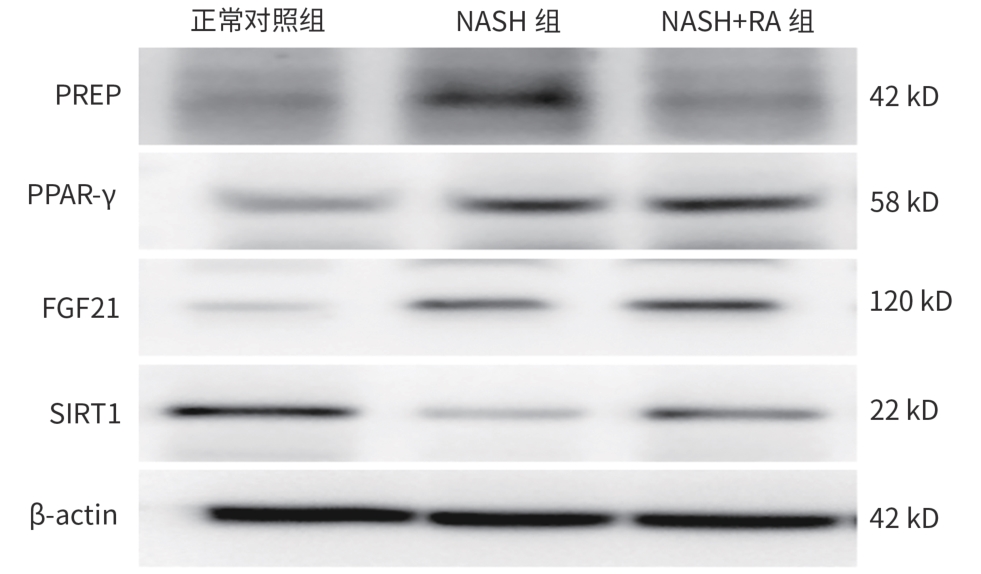
目的 探究脯氨酰内肽酶(PREP)对高脂饮食诱导的非酒精性脂肪性肝炎(NASH)小鼠模型的影响及可能的机制。 方法 将18只健康6~8周龄雄性C57BL/6J小鼠随机分为正常对照组、NASH组和NASH+迷迭香酸(RA)组,每组6只。正常对照组饲予普通饲料16周,NASH组及NASH+RA组饲予高脂饮食16周,第9周NASH+RA组予加PREP抑制剂RA灌胃,100 mg/kg,1次/d,干预8周。造模和干预结束后处死小鼠,检测不同组别小鼠的血清炎症指标、肝脏甘油三酯(TG)浓度,观察肝脏脂质、炎症、肝纤维化的改变,并计算NAFLD活动度(NAS)积分,应用Western Blot、荧光定量PCR法分别检测各组小鼠肝组织PREP、过氧化物酶体增殖物激活受体-γ(PPAR-γ)、成纤维细胞生长因子21(FGF21)、沉默信息调节因子1(SIRT1)蛋白和mRNA水平。正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t和Dunnett’s-T3检验。不满足正态分布的计量资料多组间比较及进一步两两比较采用Kruskal-Wallis H检验。 结果 NASH+RA组小鼠血清IL-6、TNF-α、TG水平均较NASH组显著降低(P值均<0.05),NASH+RA组肝脂肪变性、肝细胞水肿较NASH组明显减轻,炎细胞浸润较NASH组减少,肝组织病变明显好转。NASH+RA组NAS积分与NASH组相比显著降低(P<0.05);NASH组血管周围胶原纤维增多,偶见纤维桥连,NASH+RA组的肝脏纤维化与NASH组比较,血管周围胶原纤维稍减少。NASH组肝脏胶原面积百分比较正常对照组明显升高(P<0.05),而NASH+RA组胶原面积百分比较NASH组无明显下降(P>0.05)。NASH+RA组较NASH组PPAR-γ、FGF21、SIRT1蛋白相对表达量显著升高(P值均<0.05),而PREP蛋白相对表达量较NASH组明显降低(P<0.05)。NASH+RA组较NASH组PPAR-γ mRNA、FGF21 mRNA、SIRT1 mRNA相对表达量明显升高(P值均<0.05),PREP mRNA相对表达量较NASH组显著下降(P<0.05)。 结论 PREP通过调控PPAR-γ-FGF21-SIRT1信号通路降低炎症水平,改善小鼠NASH。 -
关键词:
- 非酒精性脂肪性肝病 /
- 脯氨酰寡肽酶类 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the effect and possible mechanism of prolyl endopeptidase (PREP) on a mouse model of non-alcoholic steatohepatitis (NASH) induced by high-fat diet. Methods A total of 18 healthy male C57BL/6J mice, aged 6 — 8 weeks, were randomly divided into normal control group, NASH group, and NASH+rosmarinic acid (RA) group, with 6 mice in each group. The mice in the control group were fed with normal diet for 16 weeks, and those in the NASH group and the NASH+RA group were fed with high-fat diet for 16 weeks; the mice in the NASH+RA group were given the PREP inhibitor RA by gavage since week 9 at a dose of 100 mg/kg, once a day for 8 weeks. The mice were sacrificed after modeling and intervention, and each group of mice was observed in terms of serum inflammatory indicators, the concentration of triglyceride in the liver, and the changes in liver lipids/inflammation/liver fibrosis; NAFLD activity score (NAS) was calculated. Western blot and quantitative real-time PCR were used to measure the protein and mRNA expression levels of PREP, peroxisome proliferator-activated receptor-γ (PPAR-γ), fibroblast growth factor 21 (FGF21), and silent information regulator 1 (SIRT1) in liver tissue. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, while the least significant difference t-test and the Dunnett’s-T3 test were used for further comparison between two groups. The Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups and further comparison between two groups. Results Compared with the NASH group, the NASH+RA group had significant reductions in the serum levels of interleukin-6, tumor necrosis factor-α, and triglyceride (all P<0.05), as well as significant improvements in hepatic steatosis, hepatocyte edema, inflammatory cell infiltration, and liver tissue lesion. The NASH+RA group had a significant reduction in NAS compared with the NASH group (P<0.05), and the NASH group had an increase in perivascular collagen fiber with occasional fiber bridging, while the NASH+RA group had a slight reduction in perivascular collagen fiber compared with the NASH group. Compared with the normal control group, the NASH group had a significant increase in collagen area percentage in the liver (P<0.05), while the NASH+RA group had no significant reduction in collagen area percentage compared with the NASH group. Compared with the NASH group, the NASH+RA group had significant increases in the relative protein expression levels of PPAR-γ, FGF21, and SIRT1 (all P<0.05) and a significant reduction in the relative protein expression level of PREP (P<0.05). Compared with the NASH group, the NASH+RA group had significant increases in the relative mRNA expression levels of PPAR-γ, FGF21, and SIRT1 (P<0.05) and a significant reduction in the relative mRNA expression level of PREP (P<0.05). Conclusion PREP reduces the level of inflammation and improves NASH in mice by regulating the PPAR-γ/FGF21/SIRT1 signaling pathway. -
表 1 PCR引物序列
Table 1. PCR primer sequence
基因 上游(5'-3') 下游(5'-3') PREP GAGCTGTACGACTACCCCAAG CCATCATCCGACAGTGTGTTG PPAR-γ CTCCAAGAATACCAAAGTGCGA GCCTGATGCTTTATCCCCACA FGF21 CTGCTGGGGGTCTACCAAG CTGCGCCTACCACTGTTCC SIRT1 CAGCCGTCTCTGTGTCACAAA GCACCGAGGAACTACCTGAT 鼠GAPDH AGGTCGGTGTGAACGGATTTG TGTAGACCATGTAGTTGAGGTCA 表 2 三组小鼠血清IL-6、TNF-α水平比较
Table 2. Comparison of serum IL-6 and TNF-α levels in three groups of mice
组别 动物数(只) IL-6(pg/mL) TNF-ɑ(pg/mL) 正常对照组 6 8.489±3.368 14.259±2.658 NASH组 6 41.466±7.5071) 44.320±7.4301) NASH+RA组 6 14.748±3.5112) 19.432±6.6772) F值 69.005 43.516 P值 <0.001 <0.001 注:与正常对照组比较,1)P<0.05;与NASH组比较,2)P<0.05。
表 3 三组小鼠NAS积分比较
Table 3. Comparison of NAS scores in three groups of mice
组别 动物数(只) NAS积分(分) 肝细胞脂肪变性(分) 小叶内炎症(分) 肝细胞气球样变(分) 正常对照组 6 0.00(0.00~1.00) 0.00(0.00~0.00) 0.00(0.00~0.00) 0.00(0.00~1.00) NASH组 6 6.50(6.00~7.25)1) 3.00(2.75~3.00)1) 1.00(2.00~2.25)1) 2.00(2.00~2.00)1) NASH+RA组 6 2.00(0.75~2.25)2) 0.00(0.00~0.00)2) 1.00(0.00~1.25) 1.00(0.75~1.25)2) χ2值 13.816 16.615 12.914 12.535 P值 0.001 <0.001 0.002 0.002 注:与正常对照组比较,1)P<0.05;与NASH组比较,2)P<0.05。
表 4 三组小鼠肝组织PREP、PPAR-γ、FGF21和SIRT1蛋白表达水平比较
Table 4. Comparison of PREP、 PPAR-γ、 FGF21 and SIRT1 protein expression in three groups of mice
组别 动物数(只) PREP PPAR-γ FGF21 SIRT1 正常对照组 6 0.401±0.057 0.419±0.057 0.161±0.043 0.580±0.006 NASH组 6 0.686±0.0291) 0.575±0.0271) 0.359±0.0341) 0.226±0.0121) NASH+RA组 6 0.467±0.0352) 0.674±0.0341)2) 0.465±0.0111)2) 0.433±0.0131)2) F值 37.397 29.068 69.334 810.942 P值 <0.001 0.001 <0.001 <0.001 注:与正常对照组比较,1)P<0.05;与NASH组比较,2)P<0.05。
表 5 三组小鼠肝组织PREP、PPAR-γ、FGF21和SIRT1基因表达水平比较
Table 5. Comparison of PREP、 PPAR-γ、 FGF21 and SIRT1 gene expression in three groups of mice
组别 动物数(只) PREP PPAR-γ FGF21 SIRT1 正常对照组 6 1.008±0.159 1.001±0.063 1.009±0.171 1.006±0.139 NASH组 6 1.769±0.2541) 1.641±0.1951) 1.688±0.0801) 0.411±0.0781) NASH+RA组 6 1.098±0.0972) 2.288±0.1751)2) 2.393±0.1441)2) 0.928±0.0802) F值 15.692 51.316 76.820 29.476 P值 0.004 <0.001 <0.001 0.001 注:与正常对照组比较,1)P<0.05;与NASH组比较,2)P<0.05。
-
[1] RONG L, ZOU JY, RAN W, et al. Advancements in the treatment of non-alcoholic fatty liver disease(NAFLD)[J]. Front Endocrinol(Lausanne), 2023, 13: 1087260. DOI: 10.3389/fendo.2022.1087260. [2] ALMOMANI A, KUMAR P, ONWUZO S, et al. Epidemiology and prevalence of lean nonalcoholic fatty liver disease and associated cirrhosis, hepatocellular carcinoma, and cardiovascular outcomes in the United States: A population-based study and review of literature[J]. J Gastroenterol Hepatol, 2023, 38( 2): 269- 273. DOI: 10.1111/jgh.16049. [3] TANG HY, LV FR, ZHANG P, et al. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease[J]. Front Endocrinol(Lausanne), 2023, 14: 1254459. DOI: 10.3389/fendo.2023.1254459. [4] IBRAHIM MK, SIMON TG, RINELLA ME. Extrahepatic outcomes of nonalcoholic fatty liver disease: Nonhepatocellular cancers[J]. Clin Liver Dis, 2023, 27( 2): 251- 273. DOI: 10.1016/j.cld.2023.01.004. [5] PAIK JM, HENRY L, YOUNOSSI Y, et al. The burden of nonalcoholic fatty liver disease(NAFLD) is rapidly growing in every region of the world from 1990 to 2019[J]. Hepatol Commun, 2023, 7( 10): e0251. DOI: 10.1097/HC9.0000000000000251. [6] YOUNOSSI ZM, GOLABI P, PAIK JM, et al. The global epidemiology of nonalcoholic fatty liver disease(NAFLD) and nonalcoholic steatohepatitis(NASH): A systematic review[J]. Hepatology, 2023, 77( 4): 1335- 1347. DOI: 10.1097/HEP.0000000000000004. [7] LU R, LIU Y, HONG TP. Epidemiological characteristics and management of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in China: A narrative review[J]. Diabetes Obes Metab, 2023, 25( Suppl 1): 13- 26. DOI: 10.1111/dom.15014. [8] NI WJ, GENG N, BAI X, et al. An excerpt of EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunctionassociated steatotic liver disease in 2024[J]. J Clin Hepatol, 2024, 40( 8): 1567- 1574. DOI: 10.12449/JCH240810.倪文婧, 耿楠, 白雪, 等.《2024年欧洲肝病学会/欧洲糖尿病学会/欧洲肥胖症学会临床实践指南: 代谢相关脂肪性肝病的管理》摘译[J]. 临床肝胆病杂志, 2024, 40( 8): 1567- 1574. DOI: 10.12449/JCH240810. [9] BABKOVA K, KORABECNY J, SOUKUP O, et al. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors[J]. Future Med Chem, 2017, 9( 10): 1015- 1038. DOI: 10.4155/fmc-2017-0030. [10] LI MT, JIANG DX, ZHANG JB, et al. Effects of sodium valproate on the progression of non-alcoholic fatty liver disease by inhibiting prolyl endopeptidase activity[J]. J Wenzhou Med Univ, 2022, 52( 8): 657- 662. DOI: 10.3969/j.issn.2095-9400.2022.08.009.李梦婷, 蒋黛西, 张建斌, 等. 丙戊酸钠抑制脯氨酰内肽酶活性对非酒精性脂肪性肝病进展的影响[J]. 温州医科大学学报, 2022, 52( 8): 657- 662. DOI: 10.3969/j.issn.2095-9400.2022.08.009. [11] JIANG DX, ZHANG JB, LI MT, et al. Prolyl endopeptidase gene disruption attenuates high fat diet-induced nonalcoholic fatty liver disease in mice by improving hepatic steatosis and inflammation[J]. Ann Transl Med, 2020, 8( 5): 218. DOI: 10.21037/atm.2020.01.14. [12] ZHOU D, LI BH, WANG J, et al. Prolyl oligopeptidase inhibition attenuates steatosis in the L02 human liver cell line[J]. PLoS One, 2016, 11( 10): e0165224. DOI: 10.1371/journal.pone.0165224. [13] SAMUEL VT, SHULMAN GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases[J]. Cell Metab, 2018, 27( 1): 22- 41. DOI: 10.1016/j.cmet.2017.08.002. [14] WANG ZY, SUN TT, YU JJ, et al. FGF21: A sharp weapon in the process of exercise to improve NAFLD[J]. Front Biosci(Landmark Ed), 2023, 28( 12): 351. DOI: 10.31083/j.fbl2812351. [15] BARROSO LN, SALARINI J, LEITE NC, et al. Effect of fish oil supplementation on the concentration of miRNA-122, FGF-21 and liver fibrosis in patients with NAFLD: Study protocol for a randomized, double-blind and placebo-controlled clinical trial[J]. Clin Nutr ESPEN, 2023, 57: 117- 125. DOI: 10.1016/j.clnesp.2023.06.027. [16] NAN Y, XIANGLI W, ZHANG W, et al. FGF21 inhibits lipid accumulation and inflammation induced by palmitate in human hepatocytes via SIRT1 pathway[J]. Chin J Cell Mol Immunol, 2019, 35( 7): 606- 612. DOI: 10.13423/j.cnki.cjcmi.008839.南瑛, 相里伟, 张薇, 等. 成纤维细胞生长因子21(FGF21)通过SIRT1通路抑制棕榈酸酯诱导的人肝细胞脂肪堆积和炎症反应[J]. 细胞与分子免疫学杂志, 2019, 35( 7): 606- 612. DOI: 10.13423/j.cnki.cjcmi.008839. [17] WU YS. Exosomes derived from lipotoxic hepatocytes in inducing LSEC capillarization and promoting the progression of non-alcoholic fatty liver disease[D]. Zhenjiang: Jiangsu University, 2022.武艳霜. 脂毒性损伤肝细胞分泌外泌体诱导LSEC毛细血管化促进NAFLD进展[D]. 镇江: 江苏大学, 2022. [18] LI YQ, TANG WJ, ZHOU YJ. Role of intestinal microbiota and metabolites in the development, progression, and treatment of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 8): 1805- 1810. DOI: 10.3969/j.issn.1001-5256.2023.08.006.李永强, 唐文娟, 周永健. 肠道菌群及其代谢产物在非酒精性脂肪性肝病发生发展及治疗中的作用[J]. 临床肝胆病杂志, 2023, 39( 8): 1805- 1810. DOI: 10.3969/j.issn.1001-5256.2023.08.006. [19] YIN JY, WANG Q. Progress on adipokines in non-alcoholic fatty liver disease[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 1- 5. DOI: 10.3969/j.issn.1674-7380.2023.01.001.尹静亚, 王琦. 脂肪因子在非酒精性脂肪性肝病中研究进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 1): 1- 5. DOI: 10.3969/j.issn.1674-7380.2023.01.001. [20] HÖFLING C, KULESSKAYA N, JAAKO K, et al. Deficiency of prolyl oligopeptidase in mice disturbs synaptic plasticity and reduces anxiety-like behaviour, body weight, and brain volume[J]. Eur Neuropsychopharmacol, 2016, 26( 6): 1048- 1061. DOI: 10.1016/j.euroneuro.2016.02.015. [21] RAPTIS DD, MANTZOROS CS, POLYZOS SA. Fibroblast growth factor-21 as a potential therapeutic target of nonalcoholic fatty liver disease[J]. Ther Clin Risk Manag, 2023, 19: 77- 96. DOI: 10.2147/TCRM.S352008. [22] YANG XN, JIN ZQ, LIN DF, et al. FGF21 alleviates acute liver injury by inducing the SIRT1-autophagy signalling pathway[J]. J Cell Mol Med, 2022, 26( 3): 868- 879. DOI: 10.1111/jcmm.17144. [23] HAN JX, LI SW, WANG WZ, et al. SIRT1 activator E1231 alleviates nonalcoholic fatty liver disease by regulating lipid metabolism[J]. Curr Issues Mol Biol, 2023, 45( 6): 5052- 5070. DOI: 10.3390/cimb45060321. [24] CHEN H, TAN HB, WAN J, et al. PPAR-γ signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets[J]. Pharmacol Ther, 2023, 245: 108391. DOI: 10.1016/j.pharmthera.2023.108391. -



 PDF下载 ( 4578 KB)
PDF下载 ( 4578 KB)


 下载:
下载: