临床医生对《慢性乙型肝炎防治指南(2022年版)》认知与实践情况的调查
DOI: 10.12449/JCH250611
Survey on the awareness and clinical application of guidelines for the prevention and treatment of chronic hepatitis B (2022 edition) among clinicians
-
摘要:
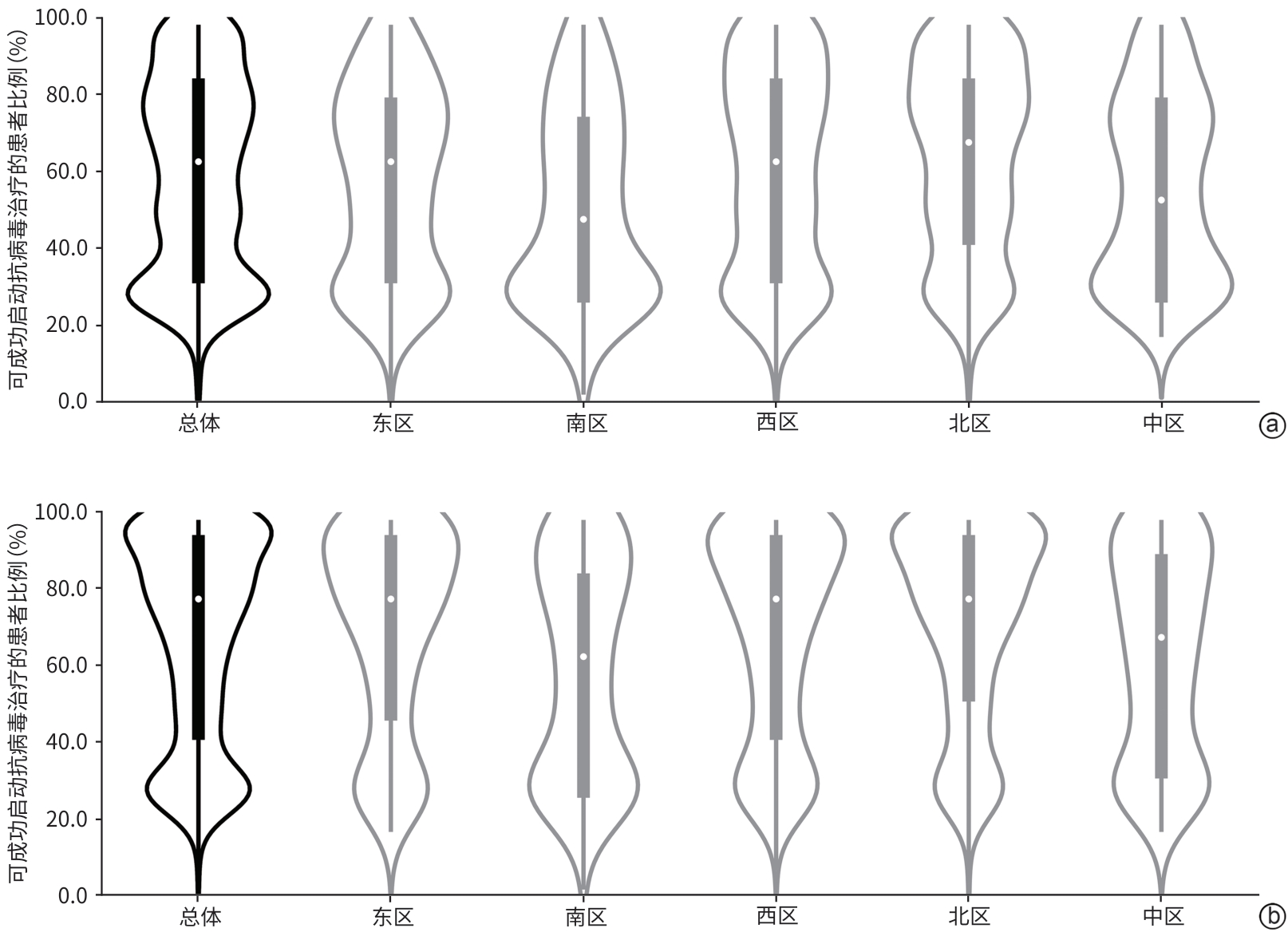
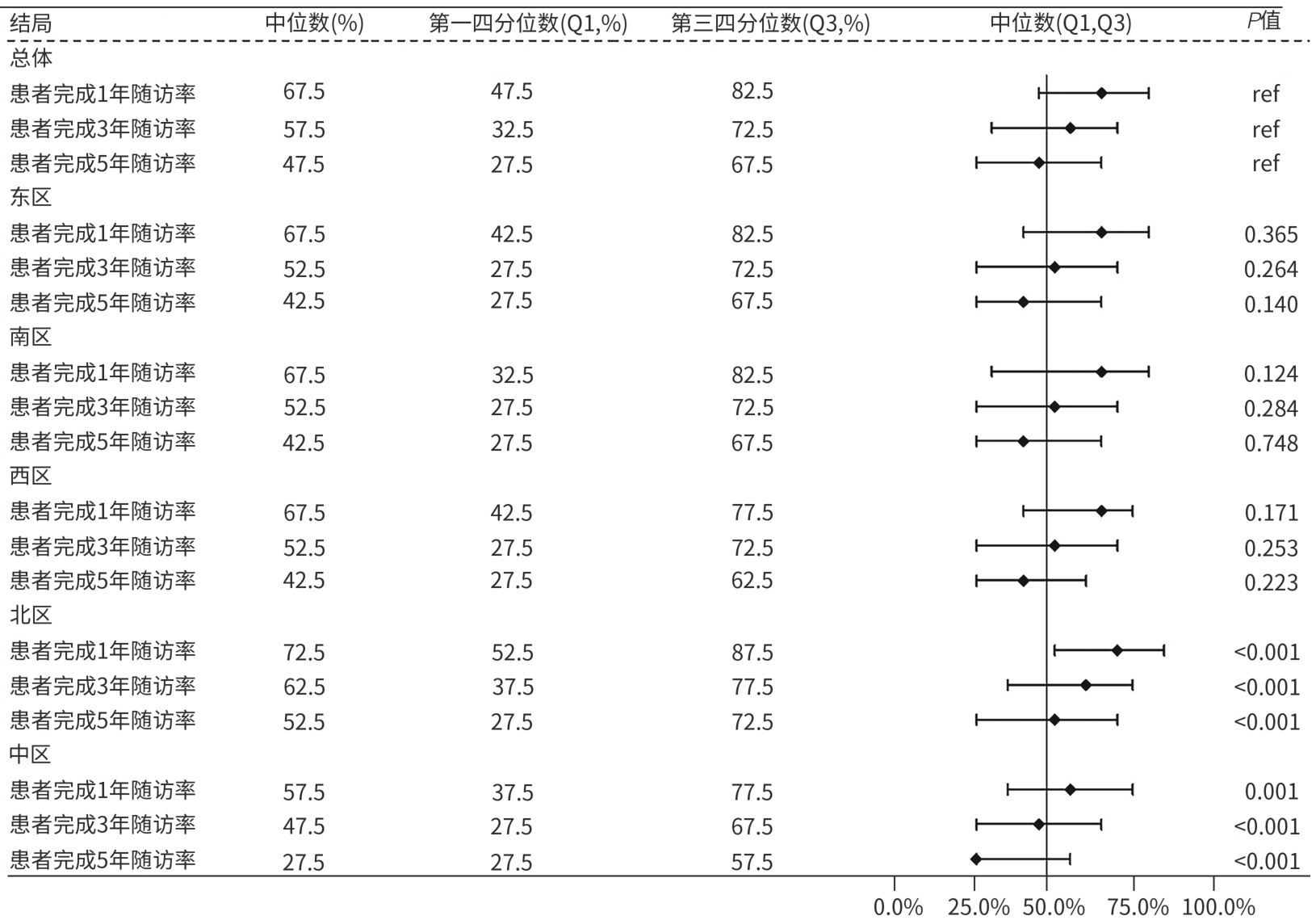
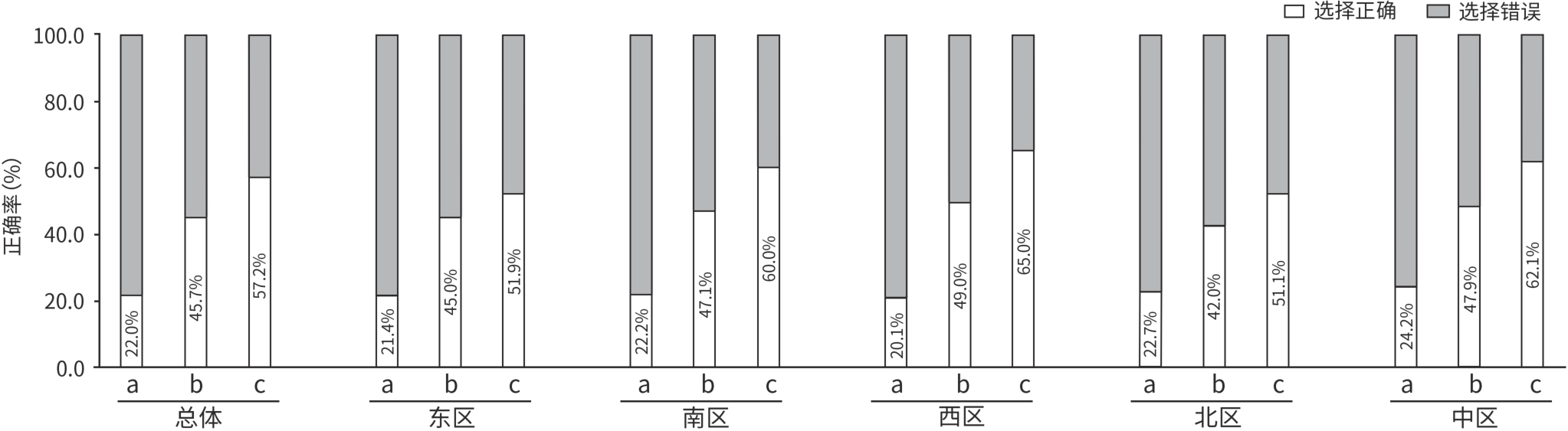
目的 评估临床医生对《慢性乙型肝炎防治指南(2022年版)》的认知及临床实践现状。 方法 2024年7月19日—12月31日采用自主编制的电子调查问卷,通过微信小程序收集了全国1 588位临床医生对检测转诊、诊治和随访三个环节共18个问题的认知和实践情况。 结果 在所有受调查者中,350位医生对《慢性乙型肝炎防治指南(2022年版)》抗病毒适应证及特殊人群治疗全部更新要点均正确知晓,总体知晓率仅为22.0%。对HBV DNA阳性、年龄>30岁者,实际进行抗病毒治疗的比例主要在20%~40%;对HBV DNA阳性、有乙型肝炎肝硬化或肝细胞癌家族史者,实际进行抗病毒治疗的比例在80%~100%。患者1、3和5年的随访率逐渐下降,中位随访率依次为67.5%、57.5%和47.5%;这与医生随访管理的时间不够,患者对于疾病认知不足,对随访的依从性不强等影响因素有关。 结论 医生对《慢性乙型肝炎防治指南(2022年版)》的认知与实践存在偏差,建议进一步加强相关培训,并重视院内慢性乙型肝炎患者“检、诊、治、管”全程规范化管理,以推动指南实践。 Abstract:Objective To investigate the awareness and clinical practice of guidelines for the prevention and treatment of chronic hepatitis B (2022 edition) among clinicians. Methods From July 19 to December 31, 2024, a self-designed electronic questionnaire was distributed via the WeChat mini program to collect related data from 1 588 clinicians nationwide, including their awareness and practice based on 18 questions regarding testing and referral, diagnosis and treatment, and follow-up. Results Among all respondents, only 350 clinicians correctly understood all the updated key points of antiviral indications and treatment for special populations in the 2022 edition of guidelines for the prevention and treatment of chronic hepatitis B, with an overall awareness rate of 22.0%. Only 20% — 40% of the patients with positive HBV DNA and an age of >30 years receive antiviral therapy, while 80% — 100% of the patients with positive HBV DNA and a family history of hepatitis B cirrhosis or hepatocellular carcinoma receive antiviral therapy. The median follow-up rates at 1 year, 3 years, and 5 years were 67.5% 57.5% and 47.5%,respectively, showing a trend of gradual reduction, which might be associated with the influencing factors such as insufficient time for follow-up management by clinicians, insufficient awareness of the disease among patients, and poor adherence to follow-up. Conclusion There is a gap between the awareness and practice of guidelines for the prevention and treatment of chronic hepatitis B (2022 edition) among clinicians. It is recommended to further strengthen training and focus on the whole process of “detection, diagnosis, treatment, and management” for patients with chronic hepatitis B in healthcare institutions, in order to promote the implementation of the guidelines. -
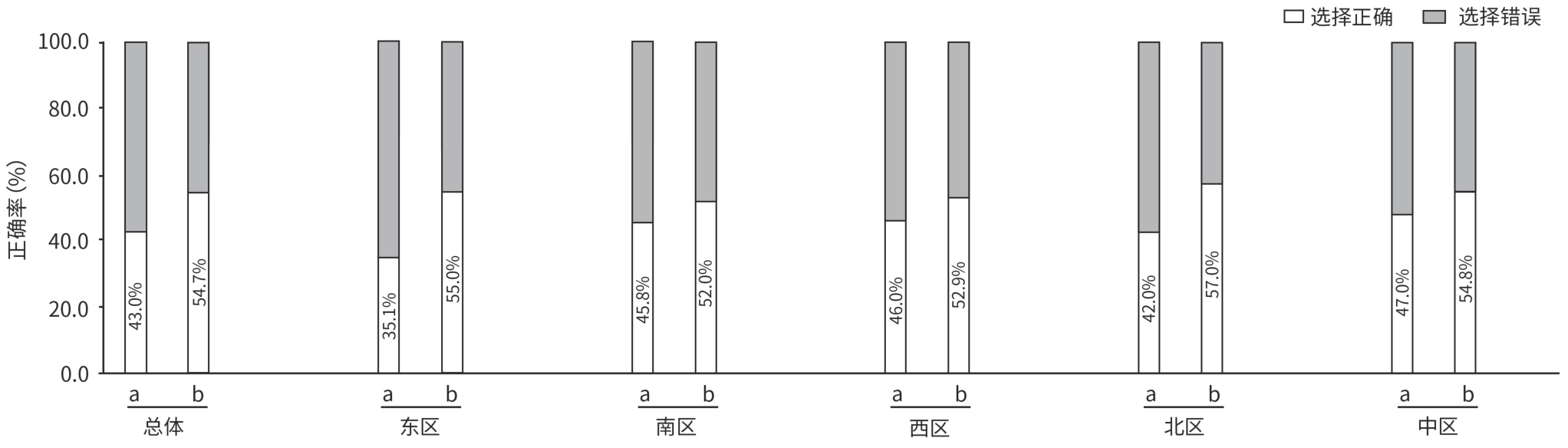
注: a,对于血清HBV DNA阳性,无论ALT水平,将年龄>30岁作为启动抗病毒治疗的独立考量因素;b,血清HBV DNA阳性,无论ALT水平,将乙型肝炎肝硬化或HCC家族史作为启动抗病毒治疗的独立考量因素;c,对于HBsAg阳性的代偿期和失代偿期乙型肝炎肝硬化患者,进行抗病毒治疗。
图 1 总体和各区医生对乙型肝炎抗病毒治疗适应证相关更新的认知情况
Figure 1. Awareness of updates on indications of antiviral therapy for chronic hepatitis B among doctors overall and by region
表 1 1 588名受调查医生的基本特征
Table 1. The characteristics of 1 588 surveyed doctors
项目 人数(例) 构成比(%) 区域分布 东区 265 16.7 南区 223 14.0 西区 362 22.8 北区 519 32.7 中区 219 13.8 医院分类及等级1) 综合医院 三级 557 35.1 二级 72 4.5 传染病/肝病专科医院 三级 933 58.8 二级 26 1.6 科室分布 感染科 629 39.6 肝病科 571 35.9 消化科 126 8.0 中西医结合科 70 4.4 医务科/感控科 16 1.0 其他(信息缺失或未确定) 176 11.1 学历 博士 245 15.4 硕士 619 39.0 本科 704 44.3 专科 20 1.3 职称 主任医师 370 23.3 副主任医师 425 26.8 主治医师 433 27.3 住院医师 360 22.6 注:1)本研究中的医院等级是依据国家卫生健康行政部门颁布的《医院分级管理办法》评定的级别。
表 2 院内病毒性肝炎协同管理科室及检出HBsAg阳性后的提醒与转诊模式
Table 2. Coordinated hospital departments for viral hepatitis management & alert-referral models for HBsAg-positive cases
项目 人数(例) 比例(%) 建议纳入院内病毒性肝炎协同管理科室 手术科室(如外科、妇产科等) 1 454 91.6 肾内/透析 1 438 90.6 侵入性检查及治疗科室(如内镜、导管、介入等) 1 417 89.2 血液内科 1 312 82.6 体检科 1 228 77.3 医院感染管理科 1 064 67.0 检验科 1 049 66.1 信息科 596 37.5 医务处 567 35.7 其他科室 71 4.5 检出HBsAg阳性后的提醒方式 检验科→信息科/感染管理科→医院HIS系统中设置“HBsAg阳性弹窗”提示 677 42.6 通过检验科新增报告单提示 442 27.8 检验科→信息科/感染管理科→建立独立的“HBV感染实时监控系统”自动提示 366 23.1 检验科→信息科→短信提醒提示负责医师 98 6.2 其他方式 5 0.3 检出HBsAg阳性后的转诊流程 门诊患者:首诊医师填写转诊单转诊至肝病/感染科门诊进行HBV DNA检测 968 61.0 住院患者:管床医师安排HBV DNA检测,阳性者邀请肝病/感染科会诊 927 58.4 门诊患者:首诊医师安排HBV DNA检测,阳性者转诊至肝病/感染科门诊 889 56.0 住院患者:管床医师邀请肝病/感染科会诊,HBV DNA检测阳性者转诊至专科 758 47.7 检验科上报→医院信息科或感染管理科→就诊科室和肝病/感染科→转诊或会诊 660 41.6 表 3 影响乙型肝炎患者长期随访管理的因素分析
Table 3. Factors affecting long-term follow-up management of hepatitis B patients
影响因素 人数(例) 比例(%) 医疗方面因素 与患者沟通随访管理的时间不够 1 072 67.5 缺乏简单便捷的随访管理工具 1 070 67.4 院内尚未形成完整、规范化的随访管理体系 1 018 64.1 随访管理人员配置困难 1 014 63.9 没有相关患者管理要求 391 24.6 患者方面因素 患者对于疾病认知不足,随访意识不强 1 512 95.2 自我感觉良好,自行停药 1 189 74.9 就诊距离原因 1 108 69.8 患者经济原因 803 50.6 家属反对意见 265 16.7 -
[1] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Infect Dis, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41( 1): 3- 28. DOI: 10.3760/cma.j.cn311365-20230220-00050. [2] XIE YD, FENG B, RAO HY. Interpretation of guidelines for the prevention and treatment of chronic hepatitis B(2022 edition)[J]. J Clin Hepatol, 2023, 39( 7): 1553- 1559. DOI: 10.3969/j.issn.1001-5256.2023.07.007.谢艳迪, 封波, 饶慧瑛.《慢性乙型肝炎防治指南(2022年版)》解读[J]. 临床肝胆病杂志, 2023, 39( 7): 1553- 1559. DOI: 10.3969/j.issn.1001-5256.2023.07.007. [3] Web of Science[EB/OL].[ 2025-03-19]. https://jcr.clarivate.com/jcr/search-results. https://jcr.clarivate.com/jcr/search-results [4] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [5] HUANG Y. Age and family history are both not required for initiation of antiviral therapy in patients with chronic hepatitis B[J]. Chin J Hepatol, 2022, 30( 4): 426- 428. DOI: 10.3760/cma.j.cn501113-20220320-00127.黄缘. 慢性乙型肝炎患者启动抗病毒治疗: 无需兼备年龄和家族史[J]. 中华肝脏病杂志, 2022, 30( 4): 426- 428. DOI: 10.3760/cma.j.cn501113-20220320-00127. [6] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67( 2): 370- 398. DOI: 10.1016/j.jhep.2017.03.021. [7] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [8] GANE EJ, CHARLTON MR, MOHAMED R, et al. Asian consensus recommendations on optimizing the diagnosis and initiation of treatment of hepatitis B virus infection in resource-limited settings[J]. J Viral Hepat, 2020, 27( 5): 466- 475. DOI: 10.1111/jvh.13244. [9] Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan society of hepatology guidelines for the management of hepatitis B virus infection: 2019 update[J]. Hepatol Res, 2020, 50( 8): 892- 923. DOI: 10.1111/hepr.13504. [10] LUBEL JS, STRASSER SI, THOMPSON AJ, et al. Australian consensus recommendations for the management of hepatitis B[J]. Med J Aust, 2022, 216( 9): 478- 486. DOI: 10.5694/mja2.51430. [11] REN S, WANG WJ, LU JF, et al. Effect of the change in antiviral therapy indication on identifying significant liver injury among chronic hepatitis B virus infections in the grey zone[J]. Front Immunol, 2022, 13: 1035923. DOI: 10.3389/fimmu.2022.1035923. [12] CHOI GH, KIM GA, CHOI J, et al. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation[J]. Aliment Pharmacol Ther, 2019, 50( 2): 215- 226. DOI: 10.1111/apt.15311. [13] ZHANG SH, WANG C, LIU B, et al. Cost-effectiveness of expanded antiviral treatment for chronic hepatitis B virus infection in China: An economic evaluation[J]. Lancet Reg Health West Pac, 2023, 35: 100738. DOI: 10.1016/j.lanwpc.2023.100738. [14] SHAN S, ZHAO XY, JIA JD. Comprehensive approach to controlling chronic hepatitis B in China[J]. Clin Mol Hepatol, 2024, 30( 2): 135- 143. DOI: 10.3350/cmh.2023.0412. [15] School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College; School of Public Health, Peking University; Department of Management on Infectious Diseases, Chinese Center for Disease Control and Prevention, et al. Expert consensus on the key technologies for a multi‑point trigger intelligent surveillance and early warning system for infectious diseases[J]. Natl Med J China, 2024, 104( 32): 2995- 3009. DOI: 10.3760/cma.j.cn112137-20240612-01317.中国医学科学院北京协和医学院群医学及公共卫生学院, 北京大学公共卫生学院, 中国疾病预防控制中心传染病管理处, 等. 传染病多点触发智慧化监测预警系统关键技术专家共识[J]. 中华医学杂志, 2024, 104( 32): 2995- 3009. DOI: 10.3760/cma.j.cn112137-20240612-01317. [16] Notice on issuing the National Disease Prevention and Control Action Plan( 2024-2025)[EB/OL].( 2024-05-29)[ 2025-03-19]. https://www.ndcpa.gov.cn/jbkzzx/c100014/common/content/content_17957038340-32320512.html. https://www.ndcpa.gov.cn/jbkzzx/c100014/common/content/content_1795703834032320512.html关于印发全国疾病预防控制行动方案( 2024— 2025 年) 的通知[EB/OL].( 2024-05-29)[ 2025-03-19]. https://www.ndcpa.gov.cn/jbkzzx/c100014/common/content/content_1795703834032320512.html. https://www.ndcpa.gov.cn/jbkzzx/c100014/common/content/content_1795703834032320512.html -



 PDF下载 ( 2075 KB)
PDF下载 ( 2075 KB)

 下载:
下载: