调整抗病毒治疗方案对慢性乙型肝炎低病毒血症患者预后的影响
DOI: 10.12449/JCH250609
Influence of antiviral treatment adjustment on the prognosis of chronic hepatitis B patients with low-level viremia
-
摘要:
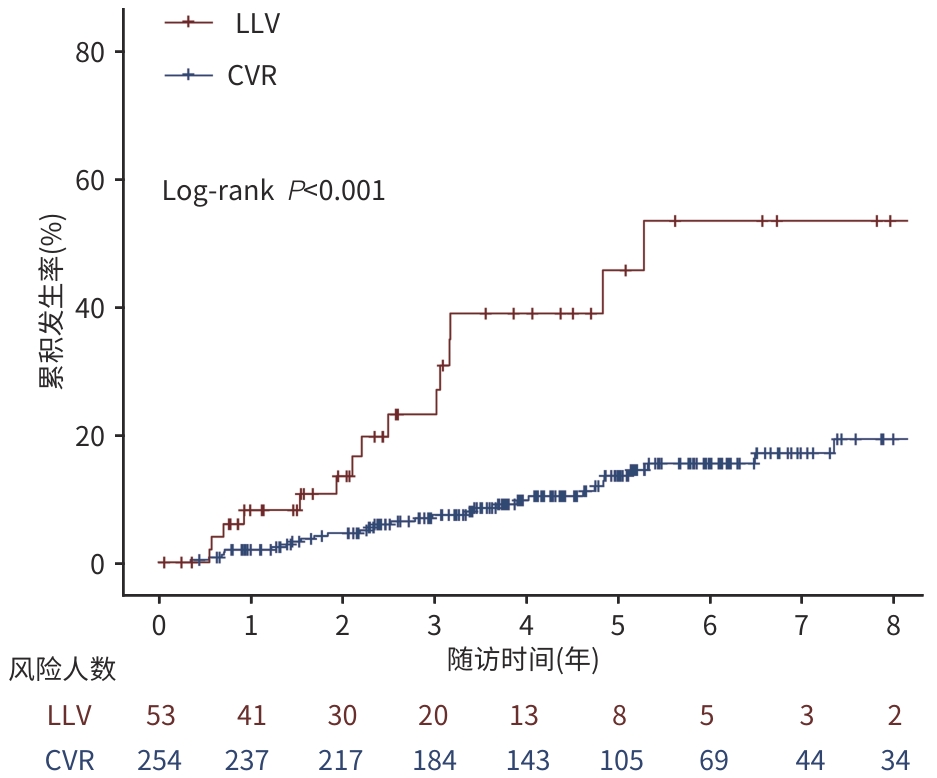
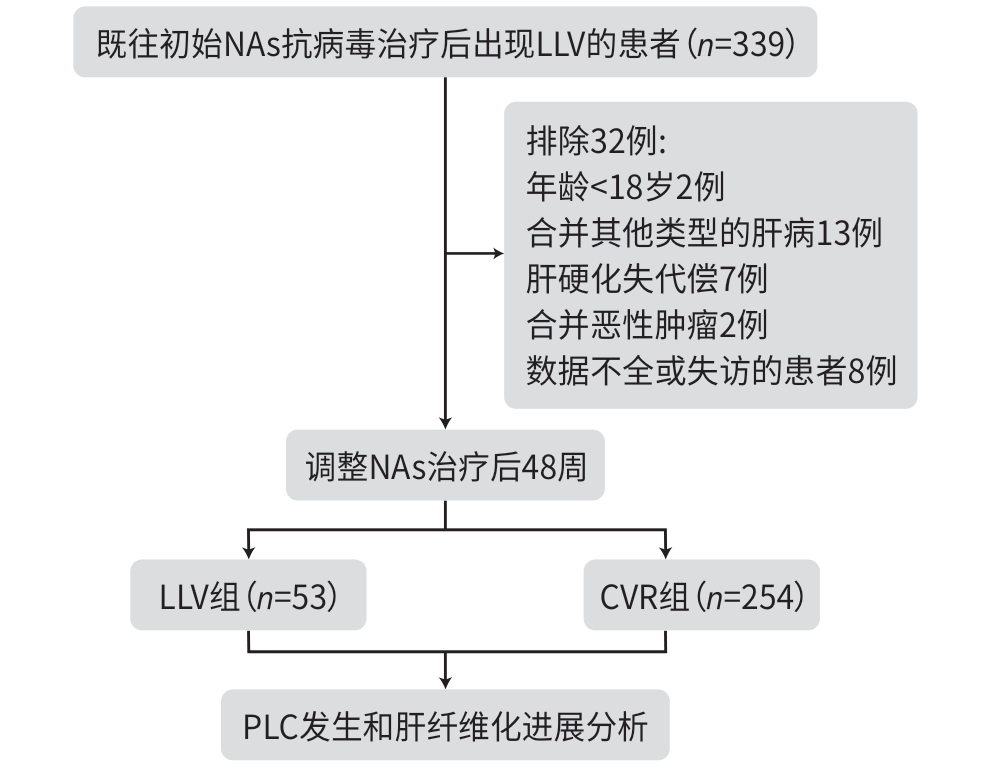
目的 探讨慢性乙型肝炎低病毒血症(LLV)(20 IU/mL≤HBV DNA<2 000 IU/mL)患者调整治疗后原发性肝癌(PLC)发生率和肝纤维化进展的情况,以期为临床实践提供更充分的证据支持。 方法 回顾性分析2007年8月—2017年4月于中国人民解放军总医院第五医学中心初始接受核苷(酸)类似物(NAs)治疗至少48周,并接受后续NAs调整的LLV患者的临床资料,根据调整治疗48周后的病毒学应答情况分为LLV组和完全病毒学应答(CVR)组(HBV DNA<20 IU/mL),每3~6个月随访1次,观察至主要终点事件PLC发生或2024年10月。观察PLC发生率和肝纤维化进展情况,肝纤维化进展定义为FIB-4分级增加≥1级。连续变量若符合正态分布两组间比较采用成组t检验;偏态分布两组间比较采用Mann-Whitney U检验。分类资料采用χ2检验进行组间比较。采用Kaplan-Meier方法计算PLC累积发生率,Log-rank法检验组间差异,采用Cox回归分析PLC的危险因素;采用Logistic回归分析肝纤维化进展的影响因素。 结果 共纳入307例患者,平均年龄为50.0岁,男性占80.5%,调整NAs方案后治疗48周时,82.7%(254例)获得CVR,17.3%(53例)仍为LLV。LLV组的PLC发生率为30.2%,肝纤维化进展率为22.6%;CVR组的PLC发生率为13.4%,肝纤维化进展率为7.5%。多因素回归分析显示,LLV是PLC发生(HR=2.623,95%CI:1.315~5.234,P=0.006)和肝纤维化进展(OR=3.213,95%CI:1.385~7.455,P=0.007)的独立危险因素。 结论 LLV一经诊断需积极调整治疗以提高CVR,若调整治疗后仍为LLV,需加强肝纤维化进展及PLC监测,以期早诊早治。 Abstract:Objective To investigate the incidence rate of primary liver cancer (PLC) and the progression of liver fibrosis in chronic hepatitis B (CHB) patients with low-level viremia (LLV) (HBV DNA<2 000 IU/mL but ≥20 IU/mL) after treatment adjustment, and to provide more robust evidence for clinical practice. Methods A retrospective analysis was performed for the clinical data of LLV patients who initially received nucleos(t)ide analogue (NAs) for at least 48 weeks at the Fifth Medical Center of PLA General Hospital from August 2007 to April 2017 and subsequently underwent NAs adjustment due to LLV, and according to the virologic response after 48 weeks of treatment adjustment, the patients were divided into LLV group and complete virological response (CVR) group (HBV DNA<20 IU/mL). The patients were followed up once every 3 — 6 months till the primary endpoint event of PLC or October 2024. The incidence rate of PLC and the progression of liver fibrosis were observed, and the progression of liver fibrosis was defined as an increase of ≥1 grade in fibrosis-4 (FIB-4) index. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of continuous data with skewed distribution between two groups; the chi-square test was used for comparison of categorical data between groups. The Kaplan-Meier method was used to calculate the cumulative incidence rate of PLC, and the Log-rank test was used for comparison between groups; the Cox regression analysis was used to investigate the risk factors for PLC, and the Logistic regression analysis was used to investigate the influencing factors for the progression of liver fibrosis. Results A total of 307 patients were enrolled, with a mean age of 50.0 years, and the male patients accounted for 80.5%. After 48 weeks of treatment with the adjusted NAs regimen, 254 patients (82.7%) achieved CVR, and 53 patients (17.3%) still had LLV. For the LLV group, the incidence rate of PLC was 30.2% and the rate of liver fibrosis progression was 22.6%, while for the CVR group, the incidence rate of PLC was only 13.4%, and the rate of liver fibrosis progression was 7.5%. The multivariate regression analyses showed that LLV was an independent risk factor for the onset of PLC (hazard ratio=2.623, 95% confidence interval [CI]: 1.315 — 5.234, P=0.006) and the progression of liver fibrosis (odds ratio=3.213, 95%CI: 1.385 — 7.455, P=0.007). Conclusion Active adjustment of treatment is needed immediately after the diagnosis of LLV to improve CVR, and if LLV persists after treatment adjustment, it is necessary to enhance the monitoring of liver fibrosis progression and PLC, so as to facilitate early diagnosis and treatment. -
Key words:
- Hepatitis B, Chronic /
- Low-level Viremia /
- Nucleos(t)ide Analogs /
- Liver Neoplasms /
- Liver Fibrosis
-
表 1 调整抗病毒治疗的方案及相应的病毒学应答情况
Table 1. Virological response under different antiviral treatment adjustment
NAs治疗调整方案 例数 CVR[例(%)] ADV+LAM/LDT→ETV+TDF/TAF 44 38(86.4) ETV/LAM/LDT→ETV+TDF/TAF 99 84(84.8) ETV/LAM/LDT→TDF/TAF 164 132(80.5) 表 2 调整治疗前LLV组与CVR组临床特征比较
Table 2. Comparison of clinical characteristics between LLV group and CVR group before adjusting treatment
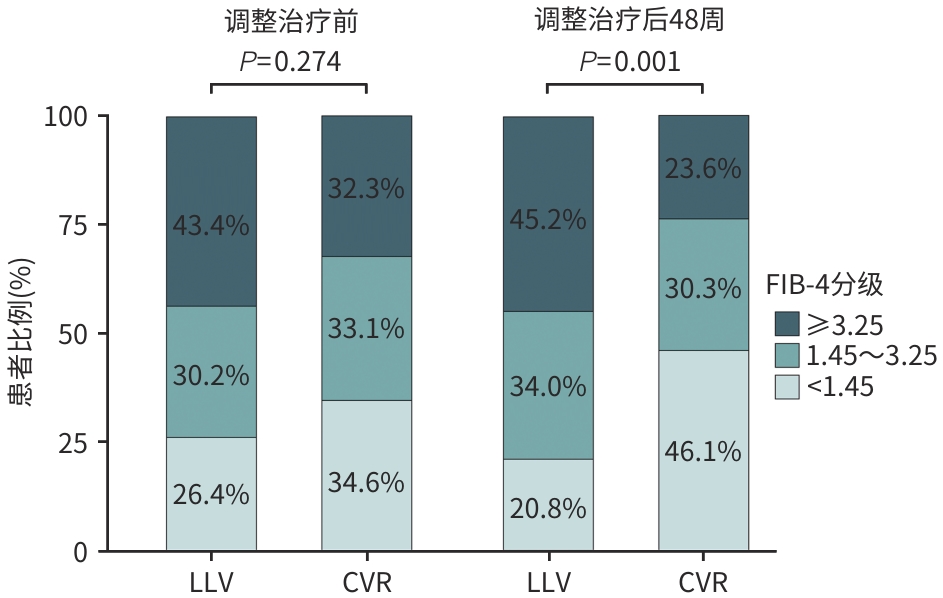
变量 总计(n=307) LLV组(n=53) CVR组(n=254) 统计值 P值 年龄(岁) 50.0±11.5 52.3±9.9 49.5±11.7 t=1.983 0.051 男性[例(%)] 247(80.5) 43(81.1) 204(80.3) χ2<0.001 >0.999 PLT(×109/L) 134.0(76.5~195.0) 121.0(70.0~180.0) 138.5(79.8~196.8) Z=-1.486 0.137 ALT(U/L) 27.0(19.0~41.0) 27.0(21.0~37.0) 27.0(19.0~41.0) Z=0.128 0.898 AST(U/L) 28.0(21.0~43.0) 34.0(25.0~58.0) 28.0(21.0~42.5) Z=1.362 0.173 TBil(μmol/L) 14.3(10.3~20.1) 14.9(9.9~21.8) 14.2(10.4~19.8) Z=0.281 0.779 HBeAg阳性[例(%)] 188(61.2) 35(66.0) 153(60.2) χ2=0.401 0.526 HBV DNA(IU/mL) 287.0(100.0~827.5) 279.0(114.0~1 070.0) 290.5(100.0~756.8) Z=0.669 0.503 qHBsAg(IU/mL) 5 196.0(3 232.5~6 699.5) 4 759.0(3 273.0~6 606.0) 5 312.5(3 183.8~6 727.0) Z=-0.376 0.707 FIB-4[例(%)] χ2=2.589 0.274 <1.45 102(33.2) 14(26.4) 88(34.6) 1.45~3.25 100(32.6) 16(30.2) 84(33.1) ≥3.25 105(34.2) 23(43.4) 82(32.3) 表 3 调整治疗48周后LLV组与CVR组临床特征比较
Table 3. Comparison of clinical characteristics between LLV group and CVR group after 48 weeks of adjusted treatment
变量 总计(n=307) LLV组(n=53) CVR组(n=254) 统计值 P值 年龄(岁) 51.0±11.5 53.3±9.9 50.5±11.7 t=1.983 0.051 男性[例(%)] 247(80.5) 43(81.1) 204(80.3) χ2<0.001 >0.999 PLT(×109/L) 162.0(94.0~210.5) 123.0(61.0~181.0) 170.0(103.8~215.8) Z=-3.043 0.002 ALT(U/L) 24.0(18.0~33.50) 29.0(21.0~40.0) 23.0(17.0~32.0) Z=2.966 0.003 AST(U/L) 25.0(21.0~34.0) 34.0(25.0~58.0) 24.0(20.0~32.0) Z=4.584 <0.001 TBil(μmol/L) 13.1(9.7~19.7) 14.8(10.2~21.5) 12.9(9.7~19.4) Z=0.831 0.406 HBeAg阳性[例(%)] 153(49.8) 40(75.5) 113(44.5) χ2=15.622 <0.001 HBV DNA(IU/mL) 0(0~0) 553.0(115.0~4 690.0) 0(0~0) Z=17.194 <0.001 qHBsAg(IU/mL) 4 783.0(1 588.0~6 478.5) 6 145.0(4 462.0~7 326.0) 4 237.0(1 482.0~6 363.0) Z=3.605 <0.001 FIB-4[例(%)] χ2=14.444 0.001 <1.45 128(41.7) 11(20.8) 117(46.1) 1.45~3.25 95(30.9) 18(34.0) 77(30.3) ≥3.25 84(27.4) 24(45.2) 60(23.6) 表 4 PLC发生风险的单因素和多因素Cox回归分析
Table 4. Univariable and multivariable Cox analysis for PLC risk
变量 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 男性 1.240(0.601~2.557) 0.561 年龄(岁) 1.047(1.019~1.076) 0.001 1.042(1.011~1.074) 0.007 PLT(×109/L) 0.992(0.988~0.996) <0.001 0.995(0.991~0.999) 0.027 ALT(U/L) 1.013(1.005~1.021) 0.001 1.001(0.987~1.015) 0.927 AST(U/L) 1.017(1.010~1.023) <0.001 1.010(0.998~1.022) 0.089 TBil(μmol/L) 1.008(1.003~1.012) 0.001 1.001(0.995~1.007) 0.724 HBeAg阳性 0.909(0.516~1.603) 0.742 LLV 4.451(2.404~8.240) <0.001 2.623(1.315~5.234) 0.006 qHBsAg(IU/mL) 1.000(1.000~1.000) 0.602 表 5 肝纤维化进展的单因素和多因素Logistic回归分析
Table 5. Univariable and multivariable Logistic analysis for fibrosis progression
变量 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 男性 1.294(0.475~3.524) 0.614 TBil(μmol/L) 0.995(0.976~1.014) 0.585 HBeAg阳性 1.678(0.784~3.589) 0.182 1.159(0.491~2.734) 0.737 LLV 3.620(1.634~8.018) 0.002 3.213(1.385~7.455) 0.007 qHBsAg(IU/mL) 1.000(1.000~1.000) 0.135 1.000(1.000~1.000) 0.526 -
[1] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Clin Infect Dis, 2022, 15( 6): 401- 427. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.001.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华临床感染病杂志, 2022, 15( 6): 401- 427. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.001. [2] ZHUANG H. Progress towards elimination of hepatitis B[J]. J Clin Hepatol, 2024, 40( 5): 857- 860. DOI: 10.12449/JCH240501.庄辉. 消除乙型肝炎进展[J]. 临床肝胆病杂志, 2024, 40( 5): 857- 860. DOI: 10.12449/JCH240501. [3] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [4] ZHANG Q, CAI DC, HU P, et al. Low-level viremia in nucleoside analog-treated chronic hepatitis B patients[J]. Chin Med J(Engl), 2021, 134( 23): 2810- 2817. DOI: 10.1097/CM9.0000000000001793. [5] MARTINEZ MG, BOYD A, COMBE E, et al. Covalently closed circular DNA: The ultimate therapeutic target for curing HBV infections[J]. J Hepatol, 2021, 75( 3): 706- 717. DOI: 10.1016/j.jhep.2021.05.013. [6] SHI Y, ZHENG M. Hepatitis B virus persistence and reactivation[J]. BMJ, 2020, 370: m2200. DOI: 10.1136/bmj.m2200. [7] JIN Y, SHEN YL, LI T. Research progress of low-level viremia in chronic hepatitis B patients treated with nucleos(t)ide analogues[J]. Chin Hepatol, 2024, 29( 8): 896- 899. DOI: 10.3969/j.issn.1008-1704.2024.08.004.金燚, 沈泳利, 李佟. 核苷(酸)类似物经治的慢性乙型肝炎患者低病毒血症的研究进展[J]. 肝脏, 2024, 29( 8): 896- 899. DOI: 10.3969/j.issn.1008-1704.2024.08.004. [8] YANG J, CHOI WM, SHIM JH, et al. Low level of hepatitis B viremia compared with undetectable viremia increases the risk of hepatocellular carcinoma in patients with untreated compensated cirrhosis[J]. Am J Gastroenterol, 2023, 118( 6): 1010- 1018. DOI: 10.14309/ajg.0000000000002181. [9] KIM JH, SINN DH, KANG W, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment[J]. Hepatology, 2017, 66( 2): 335- 343. DOI: 10.1002/hep.28916. [10] CHEN H, FU JJ, LI L, et al. Risk factors of low-level viremia in chronic hepatitis B patients receiving Entecavir monotherapy: A retrospective cohort study[J]. J Gastroenterol Hepatol, 2024, 39( 1): 180- 184. DOI: 10.1111/jgh.16357. [11] LI ZB, CHEN DD, JIA YF, et al. Risk factors related to low-level viraemia in chronic hepatitis B patients receiving entecavir treatment[J]. Front Cell Infect Microbiol, 2024, 14: 1413589. DOI: 10.3389/fcimb.2024.1413589. [12] Chinese Society of Liver Cancer, Chinese Anti-Cancer Association; Chinese Society of Clinical Oncology, Chinese Anti-Cancer Association; Liver Cancer Group, Chinese Society of Hepatology. Expert consensus on standardized diagnosis and treatment of primary liver cancer[J]. J Clin Hepatol, 2009, 25( 2): 83- 92.中国抗癌协会肝癌专业委员会, 中国抗癌协会临床肿瘤学协作专业委员会, 中华医学会肝病学分会肝癌学组. 原发性肝癌规范化诊治的专家共识[J]. 临床肝胆病杂志, 2009, 25( 2): 83- 92. [13] LYU GJ, CHEN C, HE MW, et al. Curative effect evaluation of compound Biejia Ruangan tablet on hepatic fibrosis of nonalcoholic fatty liver disease[J]. Infect Dis Inf, 2024, 37( 1): 1- 4. DOI: 10.3969/j.issn.1007-8134.2024.01.001.吕桂基, 陈椿, 贺梦雯, 等. 复方鳖甲软肝片对非酒精性脂肪性肝病肝纤维化的疗效评价[J]. 传染病信息, 2024, 37( 1): 1- 4. DOI: 10.3969/j.issn.1007-8134.2024.01.001. [14] CHENG QQ, YANG LX, CAI TP, et al. Influencing factors for low-level viremia and their dynamic changes in patients with chronic hepatitis B treated with nucleos(t)ide analogues for the first time[J]. J Clin Hepatol, 2022, 38( 12): 2716- 2722. DOI: 10.3969/j.issn.1001-5256.2022.12.008.程齐齐, 杨丽霞, 蔡天盼, 等. 核苷(酸)类似物初治的慢性乙型肝炎患者发生低病毒血症的影响因素及其动态变化分析[J]. 临床肝胆病杂志, 2022, 38( 12): 2716- 2722. DOI: 10.3969/j.issn.1001-5256.2022.12.008. [15] CHEN SH, WANG CY, GUO C, et al. Establishment of a nomogram model for predicting the survival of hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Hepatol, 2022, 38( 7): 1566- 1571. DOI: 10.3969/j.issn.1001-5256.2022.07.020.陈松海, 王春艳, 郭畅, 等. 预测HBV相关肝细胞癌生存的列线图模型的建立[J]. 临床肝胆病杂志, 2022, 38( 7): 1566- 1571. DOI: 10.3969/j.issn.1001-5256.2022.07.020. [16] ALLWEISS L, DANDRI M. The role of cccDNA in HBV maintenance[J]. Viruses, 2017, 9( 6): 156. DOI: 10.3390/v9060156. [17] WANG Y, LI YM, ZAI WJ, et al. HBV covalently closed circular DNA minichromosomes in distinct epigenetic transcriptional states differ in their vulnerability to damage[J]. Hepatology, 2022, 75( 5): 1275- 1288. DOI: 10.1002/hep.32245. [18] Coorerative Group of Basic Research and Experimental Diagnosis of Liver Disease, Chinese Society of Hepatology, Chinese Medical Association. Expert consensus on the clinical application of the markers of hepatitis B virus[J]. Chin J Hepatol, 2023, 31( 4): 389- 400. DOI: 10.3760/cma.j.cn501113-20230314-00113.中华医学会肝病学分会基础医学与实验诊断协作组. 乙型肝炎病毒标志物临床应用专家共识[J]. 中华肝脏病杂志, 2023, 31( 4): 389- 400. DOI: 10.3760/cma.j.cn501113-20230314-00113. [19] ZHENG RJ, LU XB. Low-level viremia in chronic hepatitis B patients treated with first-line treatment with nucleos(t)ide analogues and its treatment strategies[J]. J Clin Hepatol, 2024, 40( 5): 880- 883. DOI: 10.12449/JCH240506.郑嵘炅, 鲁晓擘. 一线核苷(酸)类似物经治的慢性乙型肝炎患者低病毒血症的发生及治疗策略[J]. 临床肝胆病杂志, 2024, 40( 5): 880- 883. DOI: 10.12449/JCH240506. [20] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10( 1): 1- 98. DOI: 10.1007/s12072-015-9675-4. [21] LIN CL, LIU CH, WANG CC, et al. Serum biomarkers predictive of significant fibrosis and cirrhosis in chronic hepatitis B[J]. J Clin Gastroenterol, 2015, 49( 8): 705- 713. DOI: 10.1097/MCG.0000000000000250. -



 PDF下载 ( 1391 KB)
PDF下载 ( 1391 KB)

 下载:
下载: